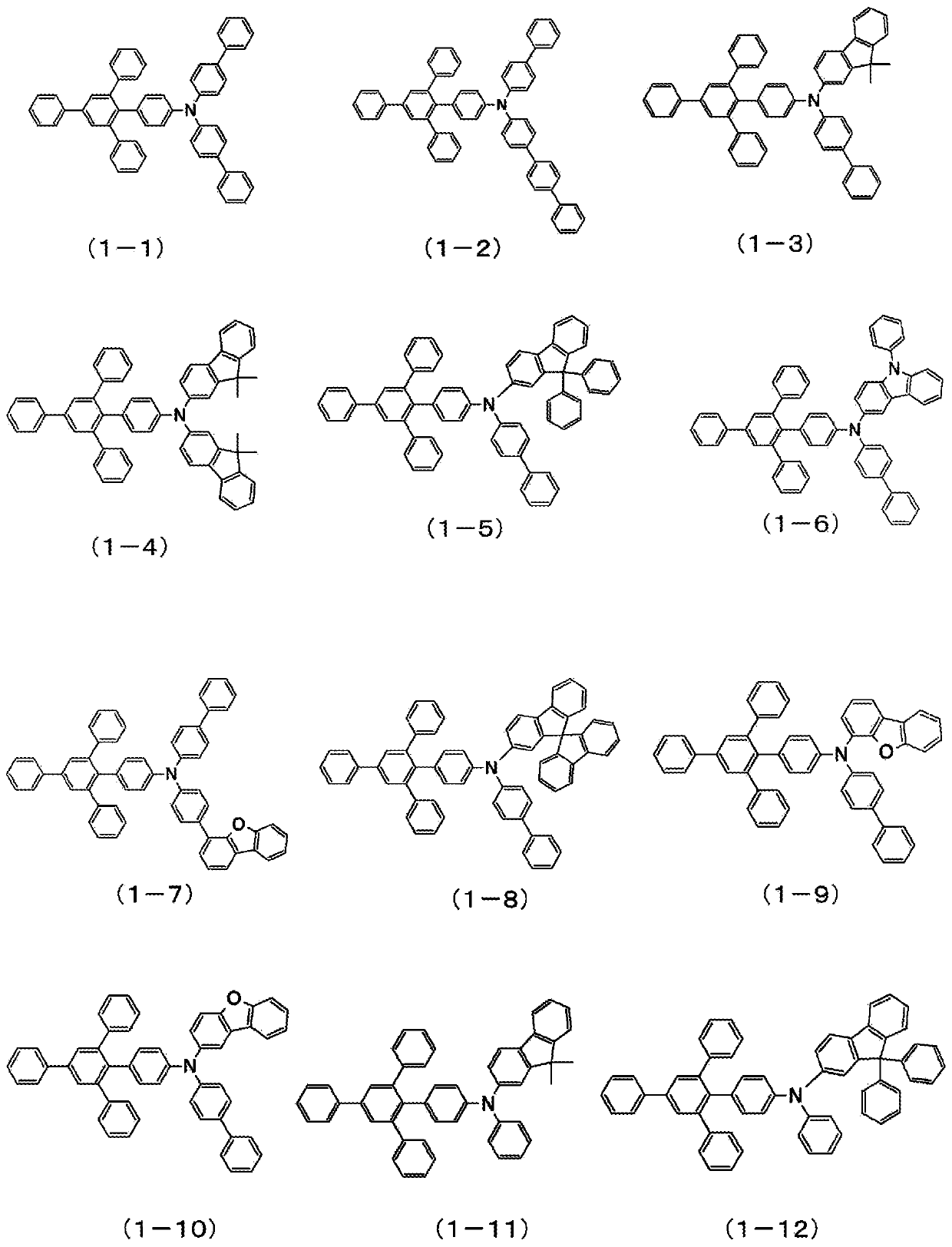
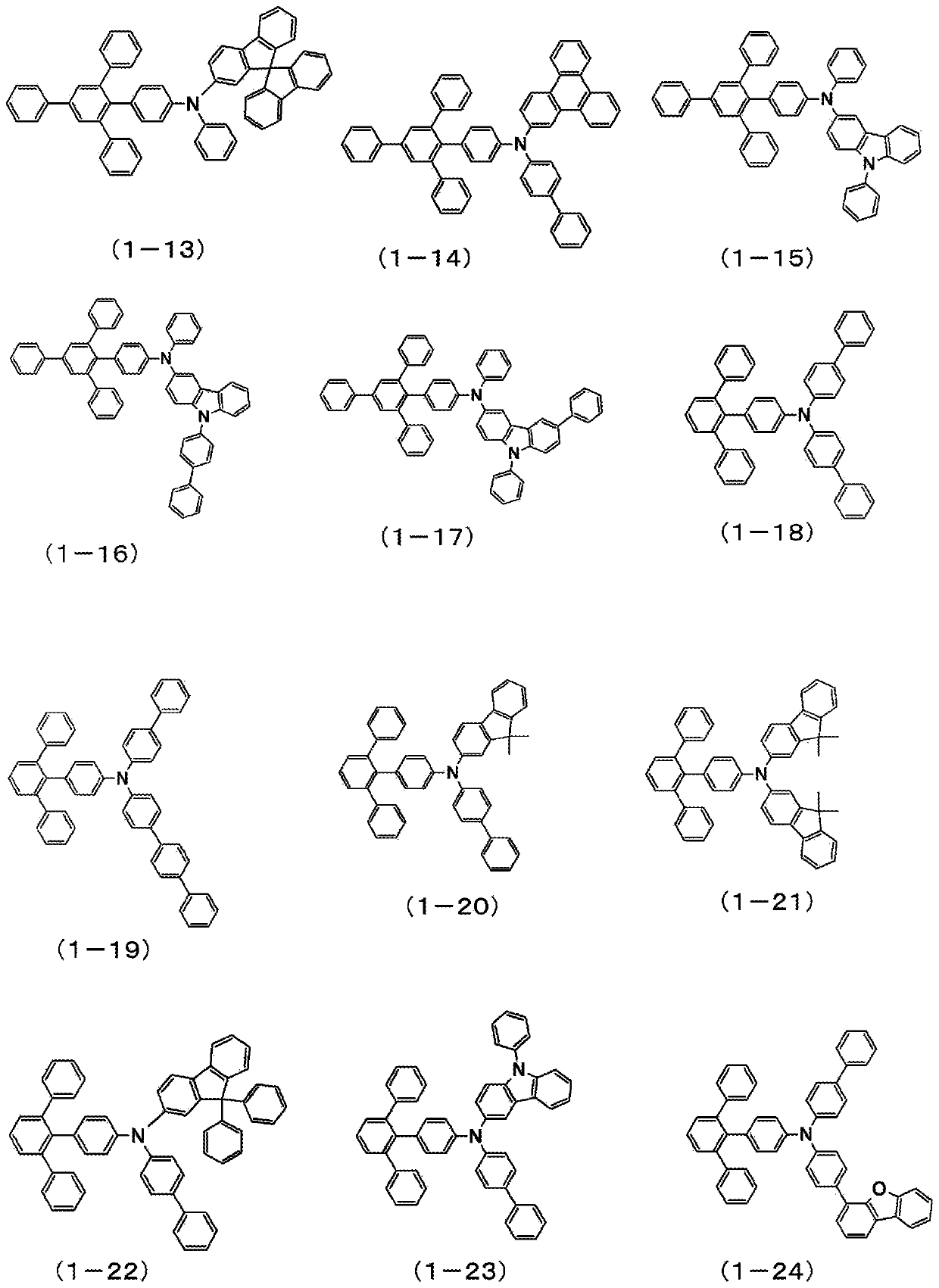
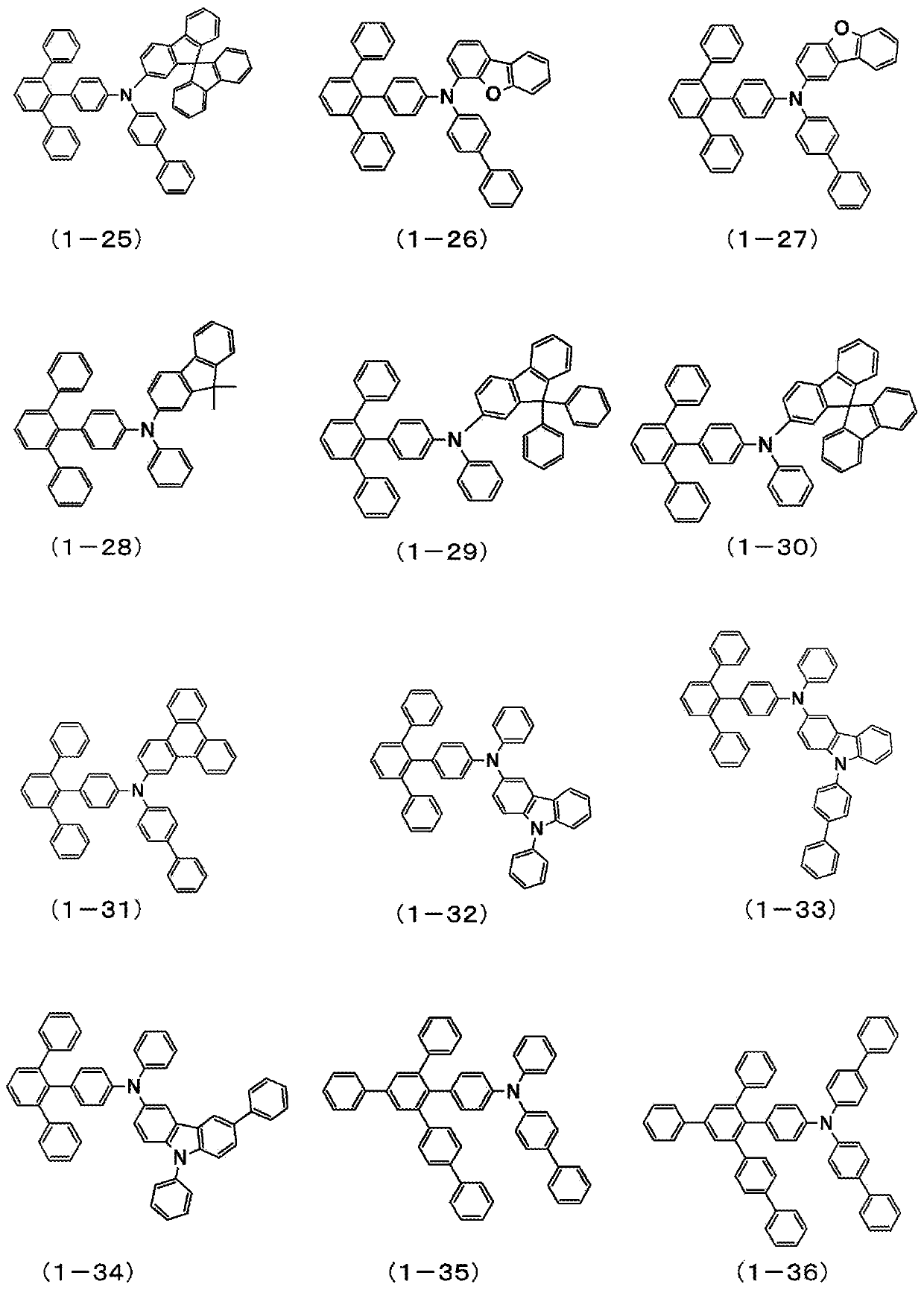
Organic electroluminescent element
An electroluminescent element, organic technology, applied in electrical elements, organic semiconductor devices, organic chemistry, etc., can solve the problems of insufficient sealing of triplet excitons, shortened component life, and reduced luminous efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0258]
[0259] After adding 50.7 g of 1,3,5-triphenylbenzene and chloroform to the nitrogen-substituted reaction container, 29.1 g of bromine was added, and it stirred at room temperature for 16 hours. After adding saturated aqueous sodium sulfite solution and stirring, liquid separation was performed to obtain an organic layer. The organic layer was dehydrated over magnesium sulfate, and concentrated under reduced pressure to obtain a crude product. Hexane was added to the crude product, and dispersion washing was performed to obtain 55.0 g of white powder of 2-bromo-1,3,5-triphenylbenzene (yield: 86%).
[0260] The obtained 2-bromo-1,3,5-triphenylbenzene 5.0g, 4-{N,N-bis(biphenyl-4-yl)amino}phenylboronic acid 6.9g, tripotassium phosphate 8.3g, 90 ml of 1,4-dioxane and 10 ml of water were added to the nitrogen-substituted reaction vessel, and nitrogen gas was passed through for 30 minutes. 0.087 g of palladium (II) acetate and 0.25 g of tricyclohexylphosphine were added,...
Embodiment 2
[0267]
[0268] 38.0 g of N-(4-bromophenyl)-4-biphenylamine, 25.5 g of 4-biphenylboronic acid, 32.4 g of potassium carbonate, 3000 ml of toluene, 76 ml of ethanol, and 113 ml of water were added to the nitrogen-substituted reaction vessel. Nitrogen was bubbled through for 30 minutes. 2.7 g of tetrakis(triphenylphosphine)palladium was added, heated, and stirred at 73° C. for 5 hours. 100 ml of water was added, and the precipitated solid was obtained by filtration. Add o-dichlorobenzene, heat and dissolve the obtained solid, add silica gel, stir, and perform hot filtration. The filtrate was concentrated under reduced pressure and the precipitated solid was obtained by filtration to give N-(biphenyl-4-yl)-N-(1,1':4',1"-terphenyl-4-yl)amine 20.1 g of yellow powder (yield 43%).
[0269] The obtained N-(biphenyl-4-yl)-N-(1,1':4',1"-terphenyl-4-yl)amine 20.0g, iodobenzene 15.4g, copper powder 0.3g, carbonic acid Add 13.9 g of potassium, 1.2 g of 3,5-di-tert-butyl salicylic acid...
Embodiment 3
[0279]
[0280]Add N-(biphenyl-4-yl)-N-(4-bromophenyl)-N-(9,9-dimethyl-9H-fluoren-2-yl)amine to a nitrogen-displaced reaction vessel 71.9g, 360ml of tetrahydrofuran, cooled to -78°C. 100 ml of a hexane solution (1.6 M) of n-butyllithium was slowly added dropwise, and stirred at the same temperature for 1 hour. Next, 19 ml of trimethyl borate was slowly added dropwise, followed by stirring at the same temperature for 1 hour. After raising the temperature to room temperature, the mixture was further stirred for 1 hour, and then, 1N aqueous hydrochloric acid solution was added thereto, followed by stirring for 1 hour. After performing a liquid separation operation to obtain an organic layer, the organic layer was dehydrated with anhydrous magnesium sulfate, and then concentrated under reduced pressure to obtain a crude product. Purify the obtained crude product by crystallization using a mixed solution of ethyl acetate / n-hexane to obtain 4-{N-(biphenyl-4-yl)-N-(9,9-dimethyl-9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com