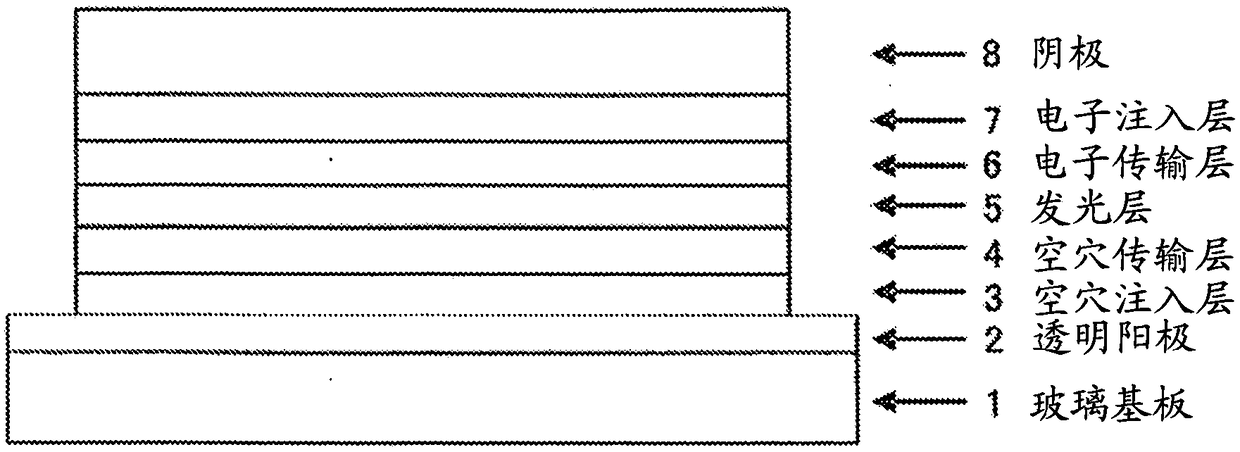
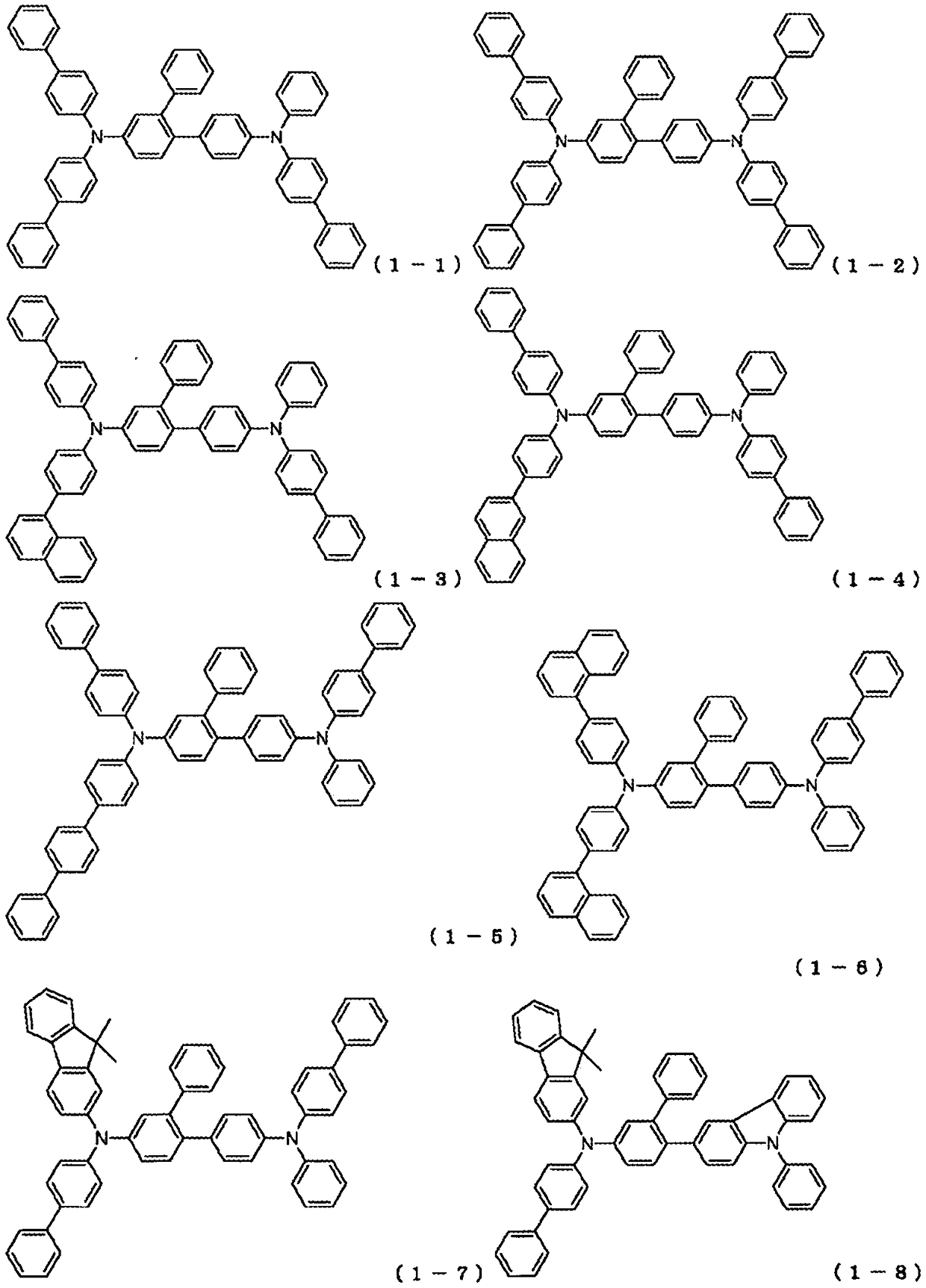
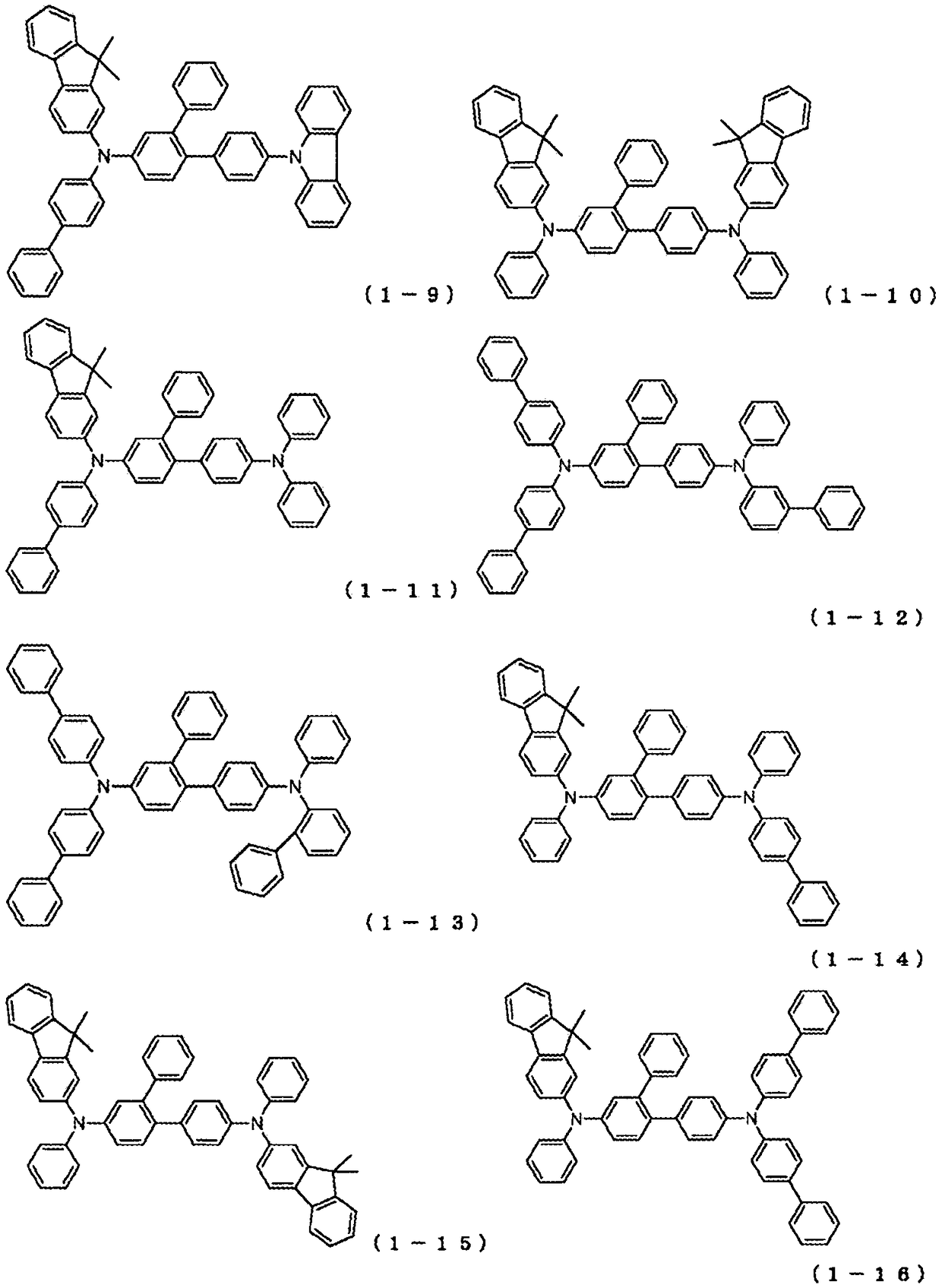
Organic electroluminescent element
An electroluminescent element and luminescent technology, applied in the direction of electrical components, electric solid-state devices, circuits, etc., can solve problems that cannot be said to be sufficient, and achieve low driving voltage, improved injection and transmission efficiency, and improved hole transport efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0387] Hereinafter, although an Example demonstrates embodiment of this invention concretely, this invention is not limited to the following Example.
Synthetic example 1
[0388]
[0389] Synthesis of 4-bis(biphenyl-4-yl)amino-4'-{(biphenyl-4-yl)-phenylamino}-2-phenyl-biphenyl:
[0390] In a nitrogen-purged reaction vessel add
[0391]
[0392]
[0393] , heated, and stirred overnight at 100°C to obtain a reaction solution. The reaction liquid was cooled, and the organic layer was extracted by a liquid separation operation. The extracted organic layer was concentrated to obtain a crude product. The crude product was purified by column chromatography (carrier: silica gel, eluent: dichloromethane / heptane). As a result, 5.30 g of white powder of compound 1-1 was obtained (yield 37%).
[0394] For the obtained white powder, the structure was identified using NMR. use 1 H-NMR (CDCl 3 ) detected the following 44 hydrogen signals.
[0395] δ(ppm)=7.65-7.60(5H)
[0396] 7.59-7.53(5H)
[0397] 7.52-7.40(9H)
[0398] 7.39-7.21(15H)
[0399] 7.20-7.10(5H)
[0400] 7.09-6.91(5H)
[0401] [chemical 14]
[0402]
Synthetic example 2
[0403]
[0404] 4-{(biphenyl-4-yl)-(4-naphthalen-1-yl-phenyl)amino}-4'-{(biphenyl-4-yl)-phenylamino}-2-phenyl- Synthesis of biphenyl:
[0405] In Synthesis Example 1, (biphenyl-4-yl)-(6-bromobiphenyl-3-yl) was used instead of bis(biphenyl-4-yl)-(6-bromobiphenyl-3-yl)amine )-(4-naphthalen-1-yl-phenyl)amine, reacted under the same conditions. As a result, 9.70 g of an off-white powder of Compound 1-3 was obtained (69% yield).
[0406] For the obtained off-white powder, use NMR to identify the structure. use 1 H-NMR (CDCl 3 ) detected the following 46 hydrogen signals.
[0407] δ(ppm)=8.07(1H)
[0408] 7.93(1H)
[0409] 7.87(1H)
[0410] 7.67-7.54(7H)
[0411] 7.54-7.11(31H)
[0412] 7.69-6.92(5H)
[0413] [chemical 15]
[0414]
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com