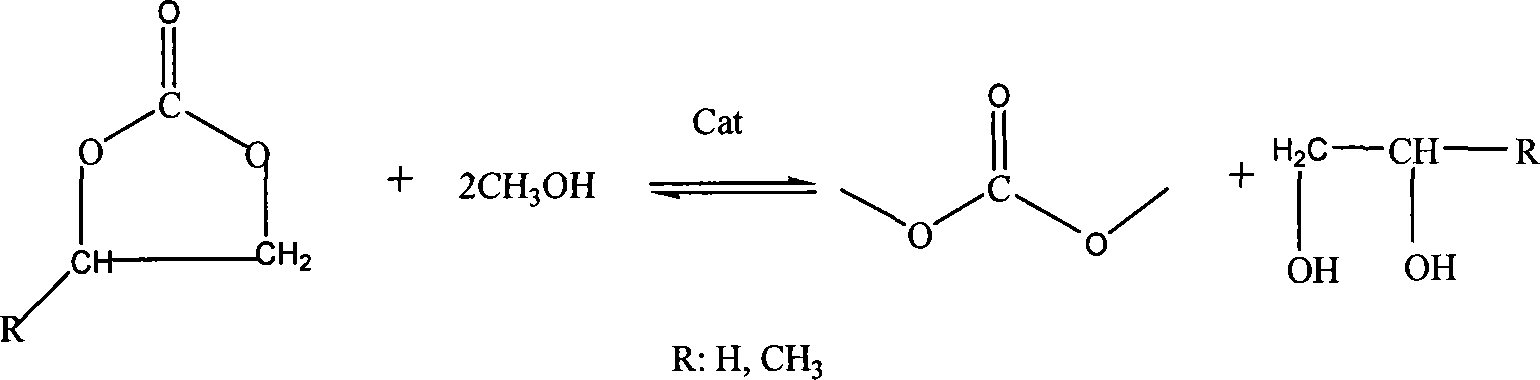
Load type solid body base catalyst of synthesizing dimethyl carbonate and method of preparing the same
A solid base catalyst, dimethyl carbonate technology, applied in the preparation of organic carbonate, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of low catalytic reaction speed, low reaction temperature, reactivity to be improved, and difficulty in repeated use. and other problems, to achieve the effects of good stability, high selectivity and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 14.17g Ca(NO 3 ) 2 4H 2 O and 11.25gAl(NO 3 ) 3 9H 2 O was dissolved in 300ml deionized water to make a mixed salt solution; weigh 5.76g NaOH and 6.36g NaOH 2 CO 3 Dissolve in 300ml deionized water to make a mixed alkali solution; pour the above two solutions into the opened fully back-mixed liquid membrane reactor at the same time, control the rotation speed of the rotor at 5000 rpm, and the reaction mixture stays in the reactor for 3 Minutes, pour the obtained slurry into a crystallization kettle, crystallize at a constant temperature of 100°C for 6 hours, then centrifuge and wash until the pH value is equal to 7, and dry the sample in an oven at 60°C for 24 hours to obtain the layered precursor Ca 2+ -Al 3+ -LDHs. The resulting layered precursor Ca 2+ -Al 3+ - LDHs were calcined in a muffle furnace at 500°C for 2 hours to obtain a particle size of 6.6nm and a specific surface area of 190m 2 / g catalyst support Ca 2+ -Al 3+ -(O).
[0031] (2) Pre...
Embodiment 2
[0038] (1) Preparation of catalyst carrier:
[0039] Weigh 38.46g Mg(NO 3 ) 2 ·6H 2 O and 18.78g Al(NO 3 ) 3 9H 2 O was dissolved in 130ml deionized water to make a mixed salt solution, and 12.8g NaOH and 10.6g NaOH were weighed 2 CO 3 Dissolve in 130ml of deionized water to make a mixed alkali solution; pour the above two solutions into the opened fully back-mixed liquid membrane reactor at the same time, the rotor speed is controlled at 8000 rpm, and the reaction mixture stays in the reactor for 2.0 Minutes, pour the obtained slurry into a crystallization kettle, crystallize at a constant temperature of 60°C for 6 hours, then centrifuge and wash until the pH value is equal to 7, and dry the sample in an oven at 60°C for 24 hours to obtain the layered precursor Mg 2+ -Al 3+ -LDHs. The resulting layered precursor Mg 2+ -Al 3+ -LDHs were calcined in a muffle furnace at 500°C for 2 hours to obtain a particle size of 6.2nm and a specific surface area of 200m 2 / g ca...
Embodiment 3
[0046] (1) Preparation of catalyst carrier:
[0047] Weigh 41.03g Mg(NO 3 ) 2 ·6H 2 O and 15.01g Al(NO 3 ) 3 9H 2 O was dissolved in 130ml deionized water to make a mixed salt solution; weigh 12.8g NaOH and 8.48g NaOH 2 CO 3 Dissolve in 130ml of deionized water to make a mixed alkali solution; pour the above two solutions into the opened fully back-mixed liquid membrane reactor at the same time, control the rotor speed at 7000 rpm, and the reaction mixture stays in the reactor for 3.0 Minutes, pour the obtained slurry into a crystallization kettle, crystallize at a constant temperature of 60°C for 6 hours, then centrifuge and wash until the pH value is equal to 7, and dry the sample in an oven at 60°C for 24 hours to obtain a layered precursor Mg 2+ -Al 3+ -LDHs. The resulting layered precursor Mg 2+ -Al 3+ - LDHs were calcined in a muffle furnace at 500°C for 2 hours to obtain a particle size of 5.6nm and a specific surface area of 210m 2 / g catalyst support Mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com