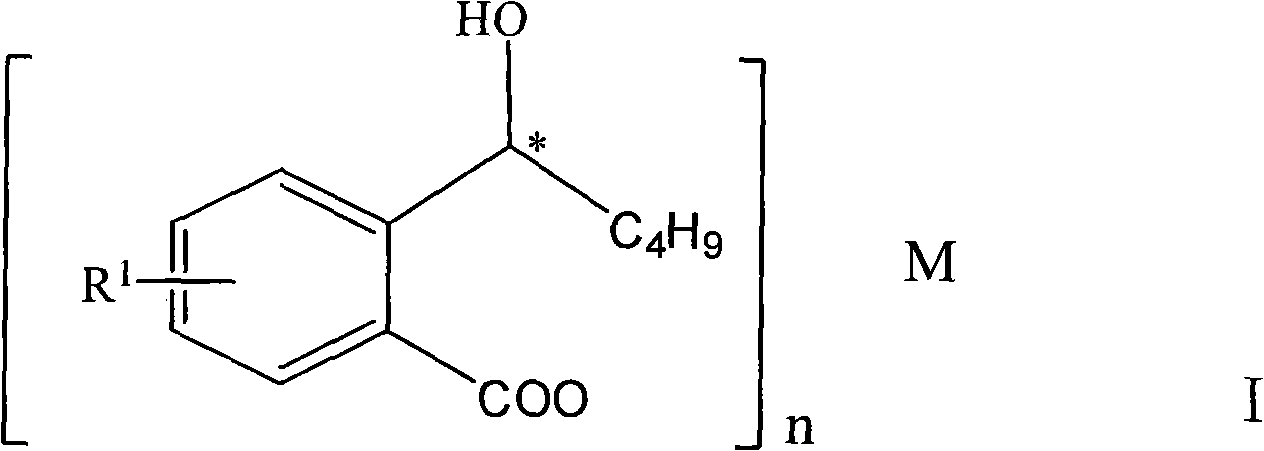
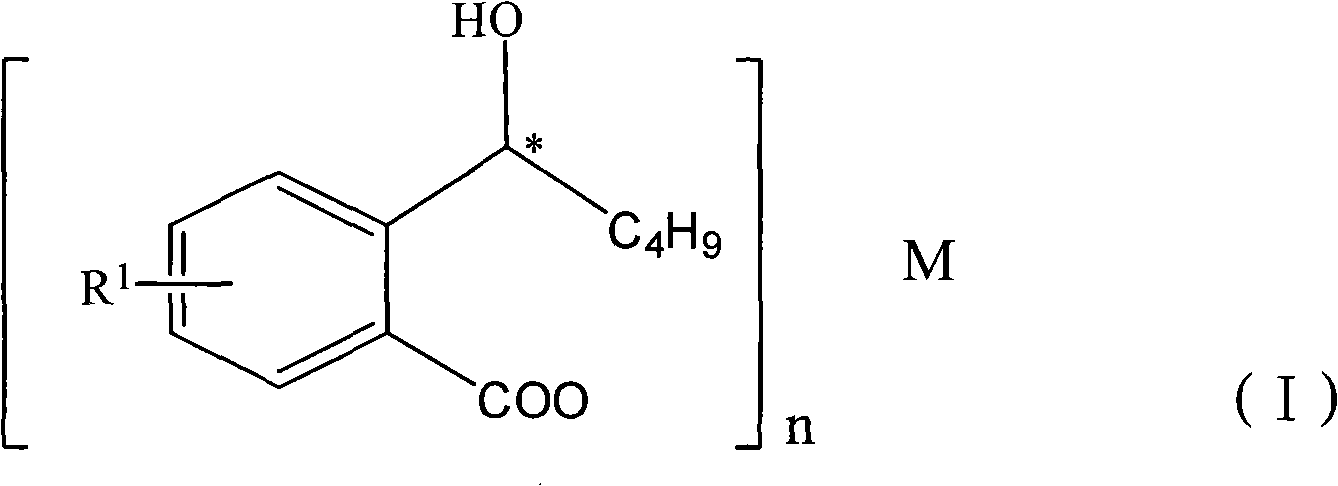
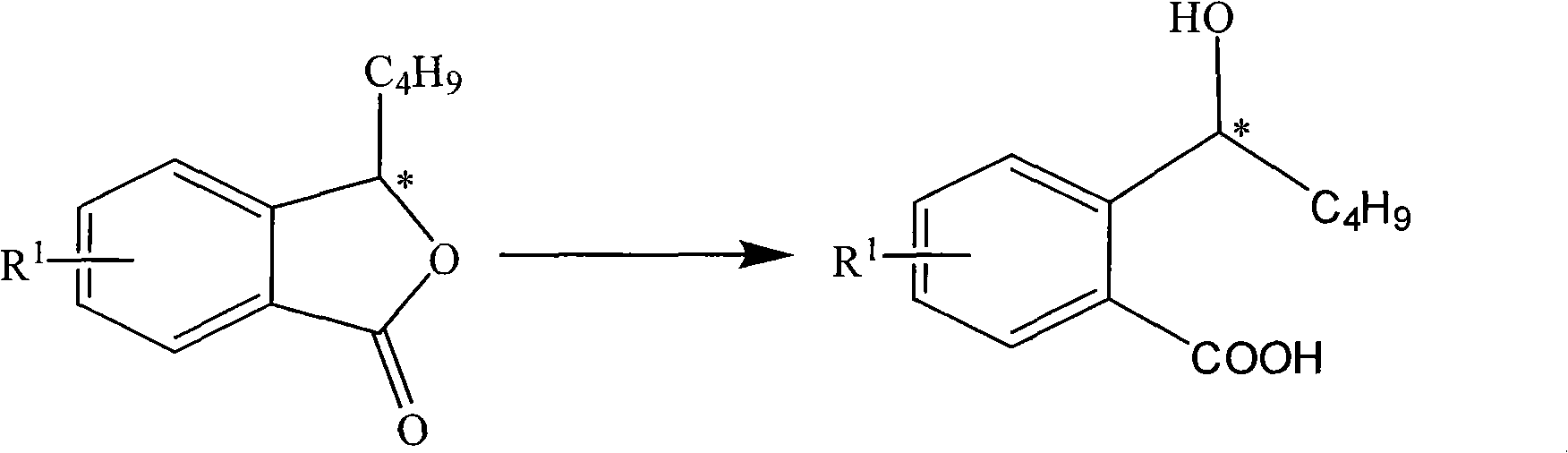
Halogenated 2-(a-hydroxyl pentyl) benzoate, production method and uses thereof
A technology of hydroxypentyl and benzoate, applied in the field of halogen-substituted 2-benzoate compounds and preparation thereof, can solve the problem of not involving the synthesis of halogenated 2-(a-hydroxypentyl)benzoate and Application and other issues, to achieve excellent anti-platelet aggregation effect, low toxicity, excellent protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of the 2-(a-hydroxypentyl) benzoic acid of halogenation
[0038]
[0039] Add halo-3-n-butylisofuran-1(3H)-one (halogenated 3-n-butylphthalide) 50g methanol 100ml, NaOH 20g and water 30ml in the reaction flask, reflux for 6 hours, remove under reduced pressure After adding methanol, add 100ml of water and cool down. Under stirring, add hydrochloric acid with a concentration of 2M dropwise to adjust the pH to 2-3. Extracted with ether, the extract was washed with water, dried, filtered, and the ether was sucked dry under reduced pressure to obtain 100 g of white solid, yield: 90%.
Embodiment 2
[0040] Embodiment 2: the preparation of halogenated 2-(a-hydroxypentyl) benzoic acid sodium salt
[0041]
[0042] Add 2.0g (0.1mol) of NaOH, 30ml of methanol, and halogenated 2-(a-hydroxypentyl)benzoic acid (0.1mol) into the reaction flask, stir at room temperature for 2 hours, add 500ml of ether in batches, and precipitate a white oil , stirred for 2 hours and left to stand, the supernatant liquid was poured out, and the oil was evaporated to dryness under reduced pressure to obtain white halogenated 2-(a-hydroxypentyl)benzoic acid sodium salt.
Embodiment 3
[0043] Embodiment 3: the preparation of halogenated 2-(a-hydroxypentyl) potassium salt of benzoate
[0044]
[0045] Dissolve 10.7g KOH (0.19mol) in 60ml of methanol, then add halogenated 2-(a-hydroxypentyl)benzoic acid (0.48mol) in batches, and finish adding in 1 hour; after adding, continue to stir for 2 hours, then Slowly pour it into 2 liters of ether solution, a white solid precipitates out immediately, after the addition is complete, continue stirring for 2 hours, filter, and dry in vacuo to obtain 54 g of a white solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com