Catalyst for synthesizing methanol through CO2 hydrogenation as well as preparation method and application
A technology for synthesizing methanol and carbon dioxide, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, preparation of hydroxyl compounds, etc., can solve the problem of low conversion rate, poor methanol selectivity, low methanol yield, etc. problem, to achieve the effect of good activity, stable performance and large quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
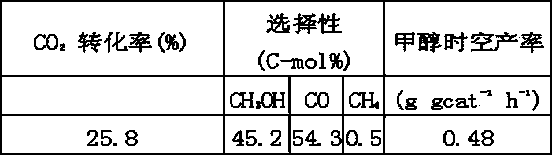
[0023] Embodiment 1. Zr is in the form of zirconyl nitrate, and all the other metal elements (Cu, Zn, Al) are dissolved in deionized water in the form of nitrate, and a mixed solution is prepared by Cu2Zn1Al0.7Zr0.3 metal atom molar ratio; at 20 Co-precipitate with a mixed solution of sodium hydroxide and sodium carbonate at ℃, the precipitation process needs to be fully stirred, keep pH = 10, age at 60 ℃ for 15 h, the precipitate is washed with deionized water until no sodium ions are detected, at 80 ℃ Dry and bake at 500°C. Then the calcined metal oxides were immersed in sodium fluoride aqueous solution (0.2 mol L –1 ), and at N 2 Stir under protection for 48 h. The resulting precipitate was filtered, CO removed 2 Multiple washes with deionized DD water (until Na-free + filter out), and at N 2 Dry at 80°C under protection, then in N 2 Calcined at 500 °C for 4 h under air protection. Broken to 40-60 mesh. The catalyst element modified by halogen can be expressed as Cu...
Embodiment 2
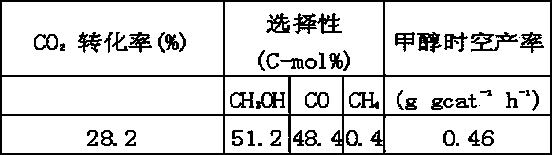
[0025] Example 2. Metal elements (Cu, Zn, Al, Y) are all dissolved in deionized water in the form of nitrate, and a mixed solution is prepared according to the molar ratio of Cu2Zn1Al0.9Y0.1 metal atoms; mixed with sodium hydroxide and sodium carbonate at 20 ° C The solution was co-precipitated, and the precipitation process required sufficient stirring to maintain pH = 8, aged at 60 °C for 15 h, the precipitate was washed with deionized water until no sodium ions were detected, dried at 80 °C and calcined at 500 °C. The calcined metal oxides were immersed in aqueous sodium fluoride (0.4 mol L –1 ), and at N 2 Stir under protection for 48 h. The resulting precipitate was filtered, CO removed 2 Multiple washes with deionized DD water (until Na-free + filter out), and at N 2 Dry at 80°C under protection, then in N 2 Calcined at 450 °C for 4 h under protection. Broken to 40-60 mesh. The halogen-modified catalyst elements can be expressed as Cu2Zn1Al0.9Y0.1F0.1 in atomic...
Embodiment 3
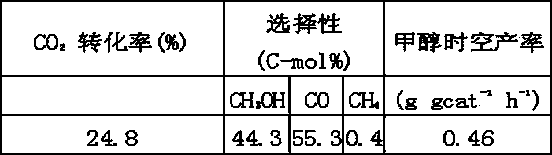
[0027]Example 3. Zr is in the form of zirconyl nitrate, the rest of the metal elements (Cu, Zn, Al) are in the form of nitrate, and the halogen fluorine is in the form of sodium fluoroaluminate, according to the atomic molar ratio of Cu2Zn1Al0.7Zr0.3F0.6 Prepare a mixed solution; co-precipitate with a mixed solution of sodium hydroxide and sodium carbonate at 20°C, fully stir during the precipitation process, keep pH = 10, age at 60°C for 15 hours, wash the precipitate with deionized water until no sodium ions are detected Drying at 80°C and firing at 500°C. The hydrogenation reaction of carbon dioxide was carried out in a stainless steel reactor with an inner diameter of 6 mm. After reduction with pure hydrogen at 350 °C for 10 h, it was cooled to room temperature and switched to reaction gas for reaction. The reaction conditions were as follows: P=5.0 MPa, T=270 °C, GHSV= 9000 mL gcat -1 h -1 , H 2 / CO 2 Molar ratio = 3, the liquid phase product was collected in an ice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com