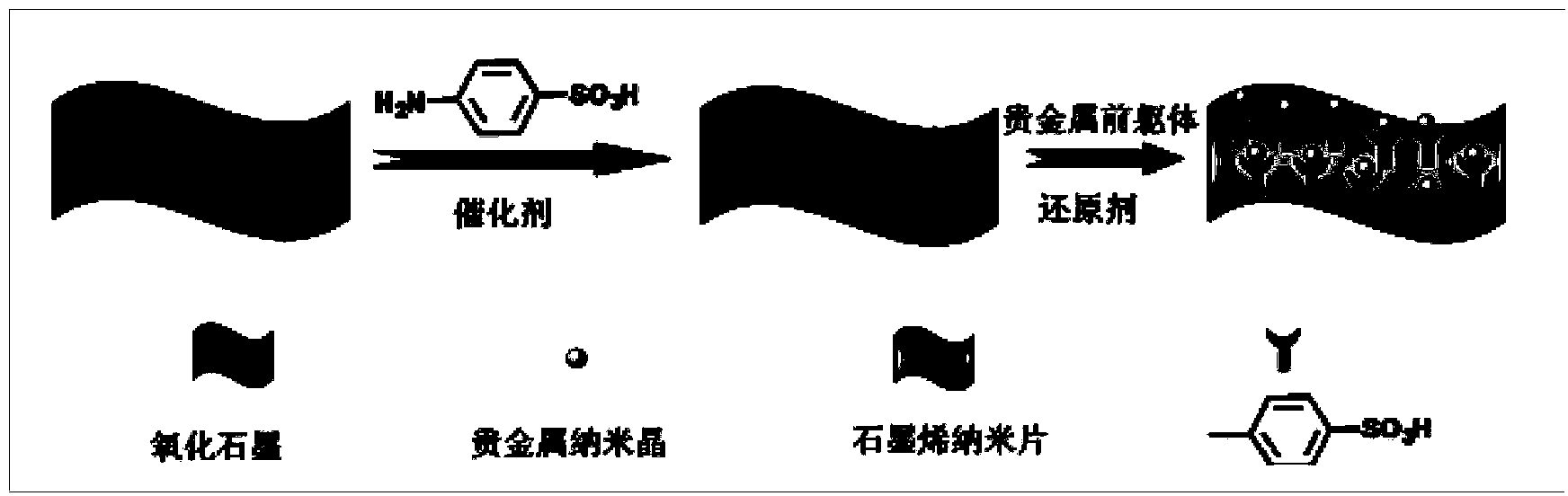
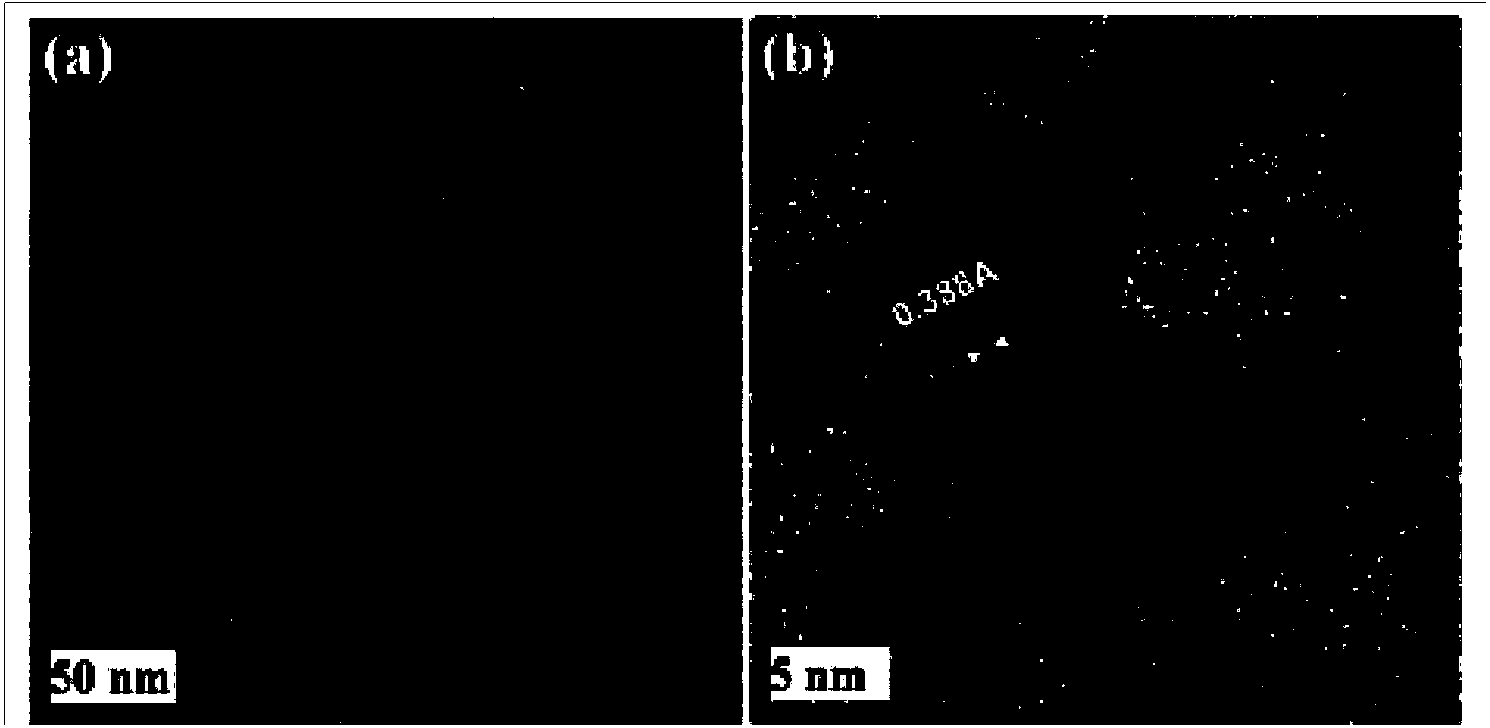
Preparation method for functionalized graphene loaded noble metal nano-crystalline composite catalyst
A noble metal nanocrystal and composite catalyst technology, which is applied in the preparation of organic compounds, the preparation of amino hydroxy compounds, and metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of uneven distribution of oxygen-containing groups and rapid aggregation. , uneven distribution of nanoparticles, etc., to achieve the effect of excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) the preparation of graphite oxide, the graphite powder of 1g is added in the reactor, the vitriol oil of 100g is added under ice-salt bath condition, graphite is dispersed evenly, then add 1g sodium nitrate and 5g potassium permanganate under stirring, After 2 hours of reaction, remove the ice-salt bath, then put the reactor into an oil bath and gradually heat to 80°C to continue the reaction for 6 hours, stop heating, and after cooling to room temperature, add hydrogen peroxide with a mass concentration of 30% until no gas is released, and then filter the product , washed with hydrochloric acid and deionized water respectively, redispersed, centrifuged, and freeze-dried to obtain graphite oxide;
[0023] (2) benzenesulfonic acid modification on graphite oxide surface, 100mg graphite oxide and 3g p-aminobenzenesulfonic acid are added to the deionized water of 100g, ultrasonic 20min, then add the sodium nitrite catalyst of 0.5g under nitrogen protection, heat to 80°C...
Embodiment 2
[0026] (1) The preparation of graphite oxide, the graphite powder of 3g is added in the reactor, the vitriol oil of 80g is added under ice-salt bath condition, graphite is dispersed evenly, then add the sodium nitrate of 2g and the permanganate of 9g under stirring Potassium, remove the ice-salt bath after 2 hours of reaction, then put the reactor into an oil bath and gradually heat it to 80°C to continue the reaction for 6 hours, stop heating, and after cooling to room temperature, add hydrogen peroxide with a mass concentration of 30% until no gas is released, and then put The product was filtered, washed with hydrochloric acid and deionized water respectively, redispersed, centrifuged, and freeze-dried to obtain graphite oxide;
[0027] (2) Benzenesulfonic acid modification on the surface of graphite oxide, add 150mg of graphite oxide to 150g of deionized water, ultrasonic for 50min, then add 1.5g of isoamyl nitrite catalyst under nitrogen protection, heat to 80°C, stir and ...
Embodiment 3
[0030] (1) The preparation of graphite oxide, the graphite powder of 2g is added in the reactor, the concentrated sulfuric acid of 90g is added under ice-salt bath condition, graphite is dispersed evenly, then add the permanganese of the sodium nitrate of 1.5g and 7g under stirring After 2 hours of reaction, the ice-salt bath was removed, and then the reactor was gradually heated to 80°C in an oil bath to continue the reaction for 6 hours, then the heating was stopped, and after cooling to room temperature, hydrogen peroxide with a mass concentration of 30% was added until no gas was released, and then The product was filtered, washed with hydrochloric acid and deionized water respectively, redispersed, centrifuged, and freeze-dried to obtain graphite oxide;
[0031] (2) Benzenesulfonic acid modification on the surface of graphite oxide, add 120mg of graphite oxide to 120g of deionized water, ultrasonic for 30min, then add 1g of sodium nitrite catalyst under nitrogen protection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com