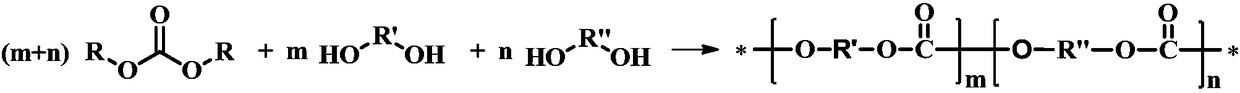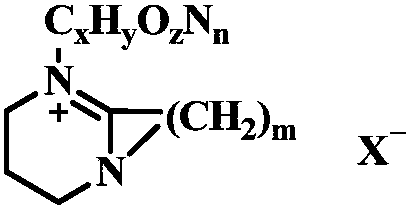Method for preparing polycarbonate catalyzed by ionic liquid
A polycarbonate, catalytic preparation technology, applied in clean catalysis, green field, can solve residual problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Implementation method: in the transesterification stage, under normal pressure, 6.85g (0.030mol) of bisphenol A and 6.75g (0.032mol) of diphenyl carbonate were heated to 150°C under a nitrogen atmosphere to melt them, and 3.18×10 -3 g (5×10 of the amount of bisphenol A substance -2 %) of acetic acidated 1,8-diazabicyclo(5,4,0)undec-7-ene, and reacted under nitrogen atmosphere for 11min to synthesize the prepolymer; -5 MPa, at a reaction temperature of 260° C., react for 20 minutes to obtain polycarbonate. After the reaction, it was cooled to room temperature under vacuum, dissolved in chloroform and precipitated in anhydrous methanol. The yield of gained polycarbonate is 97%, and molecular weight is 3.3 * 10 4 .
Embodiment 2
[0031] Same as Example 1, the catalyst used is benzoated 1,8-diazabicyclo(5,4,0) undec-7-ene 4.11×10 - 3 g (5×10 of the amount of bisphenol A substance -2 %), its structural formula is Other conditions remain unchanged, the yield of the obtained polycarbonate is 98%, and the molecular weight is 2.4×10 4 .
Embodiment 3
[0033] Same as Example 1, the catalyst used is lactated 1,8-diazabicyclo(5,4,0) undec-7-ene (3.63×10 - 3 g, 5 x 10 of the amount of bisphenol A substance -2 %), its structural formula is Other conditions remain unchanged, the yield of the obtained polycarbonate is 96%, and the molecular weight is 2.7×10 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com