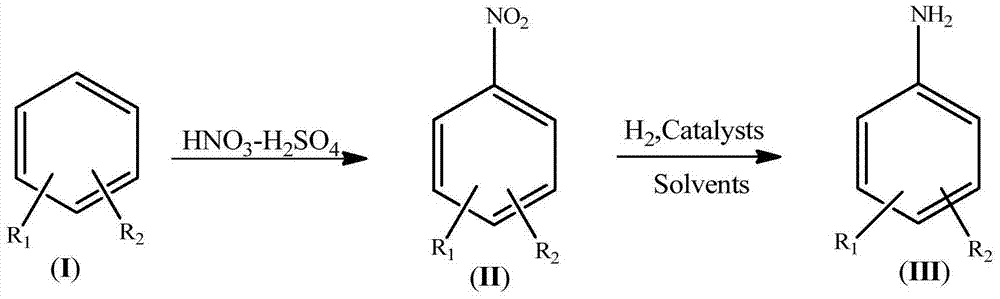
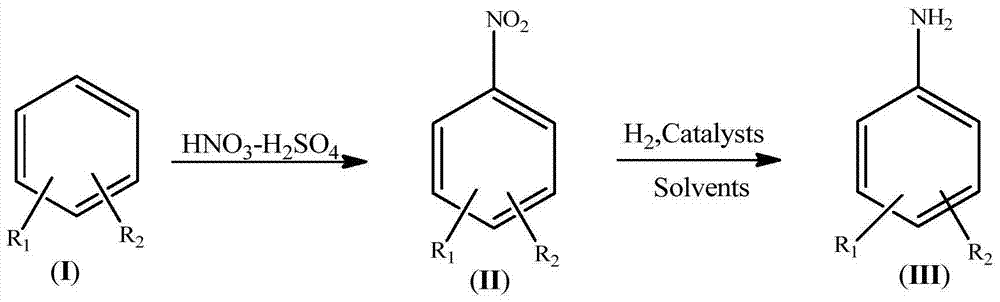
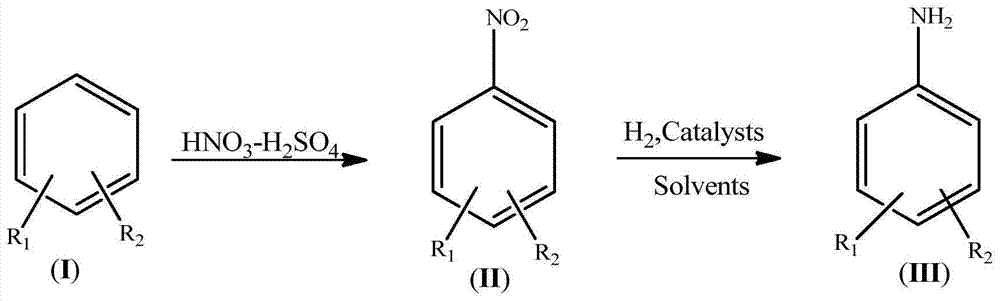
Synthetic process for amino aromatic hydrocarbon compound
A technology of aromatic compounds and aminoaromatics, which is applied in the field of synthesis of aminoaromatic compounds, can solve the problems of producing highly toxic nitrogen oxides, spending a lot of money to recover nitric acid, endangering biological health, etc., achieving fast speed, improving purity and yield , the effect of high industrial safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Preparation of nitration reaction solution:
[0049] Reaction solution-1: Weigh 78g of benzene as reaction solution-1;
[0050] Reaction solution-2: Weigh 96.9g (65%) of nitric acid and 78.8g (98%) of concentrated sulfuric acid and mix, keep the temperature of the mixing process not exceeding 15°C, and use it as reaction solution-2;
[0051] (2) Synthesis of nitrobenzene:
[0052] Keep the temperature of the micro-reactor at 20°C, add the reaction solution-1 and the reaction solution-2 into the micro-reactor through the syringe pumps-1 and -2 respectively, the flow rate of the reaction solution-1 is 20mL / min, the flow rate of the reaction solution-2 The flow rate is 22 mL / min, and at this time, the molar ratio of nitric acid to benzene added is 1.05:1. After the reaction was complete, 236g of water was added and filtered to obtain 113.2g of nitrobenzene product. The nuclear magnetic resonance spectrum data of described nitrobenzene compound is as: 1 HNMR (500MHz...
Embodiment 2
[0058] (1) Preparation of nitration reaction solution:
[0059] Reaction solution-1: weigh 165g of p-acetamidoanisole and mix with 825g (98%) concentrated sulfuric acid as reaction solution-1;
[0060] Reaction solution-2: Weigh 67.0g (95%) nitric acid as reaction solution-2;
[0061] (2) Synthesis of 2-nitro-4-acetamidoanisole:
[0062] Keep the temperature of the microreactor at 50°C, and add the reaction solution-1 and the reaction solution-2 into the microreactor through the syringe pumps-1 and -2 respectively, the flow rate of the reaction solution-1 is 10mL / min, and the flow rate of the reaction solution-2 The flow rate is 95mL / min, at this time, the molar ratio of nitric acid to benzene added is 1.01:1. After the reaction was completed, 3300 g of water was added and filtered to obtain 205.8 g of 2-nitro-4-acetamidoanisole product. The NMR spectrum data of the 2-nitro-4-acetamidoanisole compound are as follows: 1 HNMR (500MHz, CDCl 3 ): δ1.62(s,2H), 4.20(s,3H), 7.42...
Embodiment 3
[0068] Reaction solution-1: Weigh 108g of anisole as reaction solution-1;
[0069] Reaction solution-2: Weigh 63g (95%) nitric acid and 108g (98%) concentrated sulfuric acid and mix, keep the temperature during the mixing process not exceeding 15°C, and use it as reaction solution-2;
[0070] (2) Synthesis of nitroanisole:
[0071] The temperature of the microreactor was kept at 10°C, and the reaction solution-1 and the reaction solution-2 were added to the microreactor through the syringe pumps-1 and -2 respectively. The flow rate of the reaction solution-1 was 10mL / min, and the flow rate of the reaction solution-2 was The flow rate was 9.5mL / min. At this time, the molar ratio of nitric acid and anisole was 1:1. After the reaction was completed, 324g of water was added and filtered to obtain 150g of mixed nitroanisole product.
[0072] (3) Preparation of reduction reaction solution
[0073] Reaction solution-3: Mix 150g of mixed nitroanisole obtained in the previous step wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com