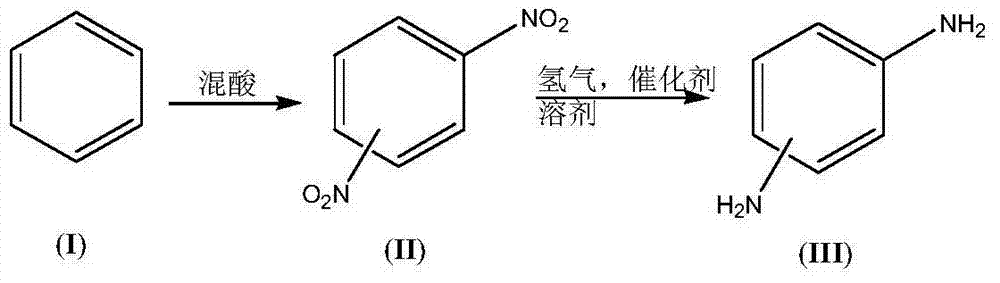
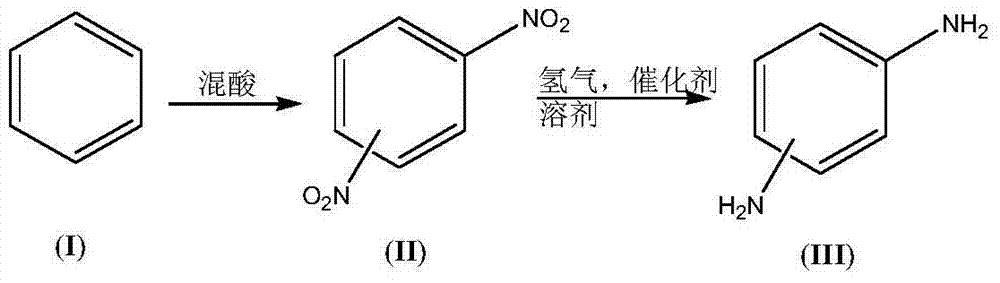
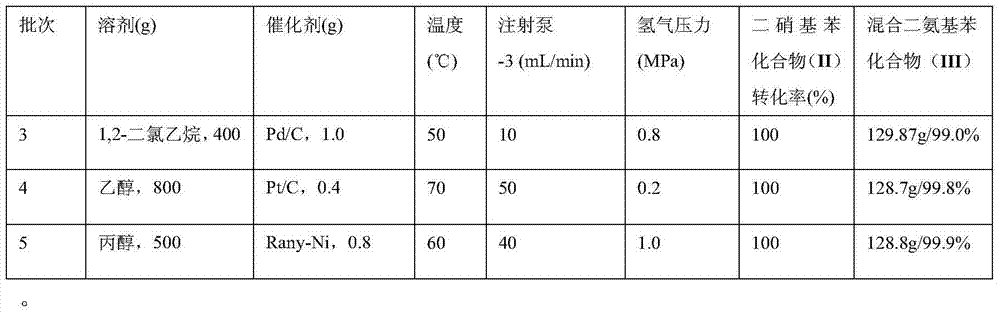
Synthesis process of dimido dipheny compound
A technique for the synthesis of diaminobenzene, which is applied in the preparation of amino compounds, the preparation of organic compounds, and organic chemistry, and can solve problems such as low industrial safety, many side reactions, and easy coking of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation of nitration reaction solution:
[0040] Reaction solution-1: weigh 78.8g (99%) benzene (I) as reaction solution-1;
[0041] Reaction solution-2: Weigh 128.5g (98%) nitric acid and 390g (98%) concentrated sulfuric acid and mix, keeping the temperature during the mixing process not exceeding 15°C, as reaction solution-2;
[0042] (2) Synthesis of dinitrobenzene compound (II):
[0043]Keep the temperature of the microreactor at 70°C, and add the reaction solution-1 and the reaction solution-2 into the microreactor through the syringe pumps-1 and -2 respectively, the flow rate of the reaction solution-1 is 20mL / min, and the flow rate of the reaction solution-2 The flow rate is 44mL / min. At this time, the molar ratio of nitric acid and benzene (I) added is 2.1:1, and the reaction is completed in about 5 minutes. The conversion rate of raw material benzene is 100%, and 162.1g (99.8%) of mixed dinitrobenzene products are obtained. (II, wherein the content of...
Embodiment 2
[0053] (1) Preparation of nitration reaction solution:
[0054] Reaction solution-1: weigh 788g (99%) benzene (I) as reaction solution-1;
[0055] Reaction solution-2: Weigh 1285.7g (98%) nitric acid and 2364g (98%) concentrated sulfuric acid and mix, keeping the temperature during the mixing process not exceeding 15°C, as reaction solution-2;
[0056] (2) Synthesis of dinitrobenzene compound (II):
[0057] Keep the temperature of the micro-reactor at 90°C, add the reaction liquid-1 and the reaction liquid-2 into the micro-reactor through the syringe pumps-1 and -2 respectively, the flow rate of the reaction liquid-1 is 20mL / min, the flow rate of the reaction liquid-2 The flow rate is 44mL / min. At this time, the molar ratio of nitric acid and benzene (I) added is 2.1:1, and the reaction is completed in about 50 minutes. The conversion rate of raw material benzene (I) is 100%, and 1646g (99.9%) of mixed dinitro Benzene compound (II, wherein the content of mononitrobenzene is ...
Embodiment 3~6
[0059] (1) Preparation of reduction reaction solution
[0060] Weigh 200 g of the mixed dinitrobenzene compound (II) synthesized in Example 2 and mix them with different solvents and different catalysts respectively, as reduction reaction solution-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com