Preparing method for nano micron lead sulfate with electrochemical activity and method adopting lead sulfate to prepare lead-acid cell
A lead sulfate, nano-micron technology, applied in lead-acid battery electrodes, nanotechnology for materials and surface science, lead sulfate, etc., can solve problems such as energy consumption, high material consumption, and high pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
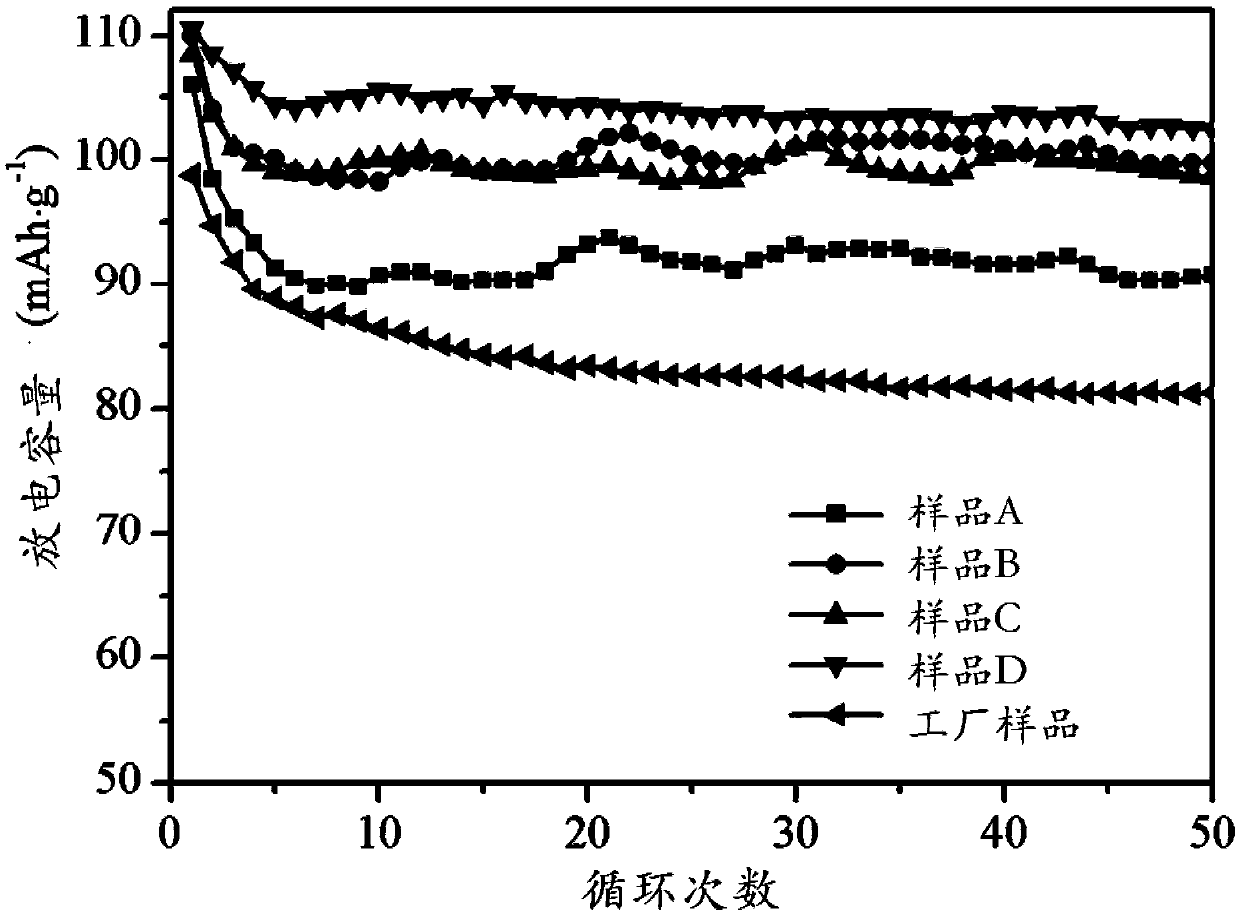
Image
Examples
Embodiment 1~3
[0060] Embodiment 1~3: the aqueous solution reaction of soluble lead salt solution and soluble sulfate / sulfuric acid
Embodiment 1
[0061] Embodiment 1: First, take lead nitrate and equimolar ammonium sulfate to be dissolved in the water of equivolume respectively, the concentration of solution is 0.1mol / L, then divide into two storage tanks. Secondly, add soluble chloride (such as NaCl, KCl, etc.) with 0.09 times the molar number of lead nitrate to the lead nitrate solution, and stir to dissolve it completely (if white precipitate is formed, the temperature can be raised to dissolve it). Then, the two solutions were mixed and reacted in a counterflow reactor, the flow rate was controlled to be 0.1L / min, and the residence time in the reactor was not more than 30 minutes, and the ultrafine PbSO 4 Separated from the mother liquor, then washed with water to remove impurity ions and then dried to obtain nano-micron PbSO 4 powder.
Embodiment 2
[0062] Embodiment 2: at first, take by weighing lead chloride and the potassium sulfate of slight excess (5% of lead chloride molarity of excess value) and be dissolved in the water of equal volume respectively, the concentration of solution is 0.05mol / L, separates then in the second storage tank. Secondly, add sodium lauryl sulfate with 0.1 times the molar number of lead chloride to the lead chloride solution, and stir to dissolve it completely, because the solubility of lead chloride is small, the temperature of the solution should be raised appropriately, but not more than 100°C. Then, the two solutions were mixed and reacted in a counterflow reactor, the flow rate was controlled to be 3L / min, the residence time in the reactor was no more than 20 minutes, and the ultrafine PbSO 4 Separated from the mother liquor, then washed with water to remove impurity ions and then dried to obtain nano-micron PbSO 4 powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com