Double-perovskite structure catalyst material for cathode of lithium air battery and preparation method of catalyst material
A structural catalyst, lithium-air battery technology, applied in battery electrodes, structural parts, fuel cell-type half-cells and primary battery-type half-cells, etc., to achieve the effects of increased discharge voltage, good repeatability, and good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Sr 2 CrMoO 6 (a)
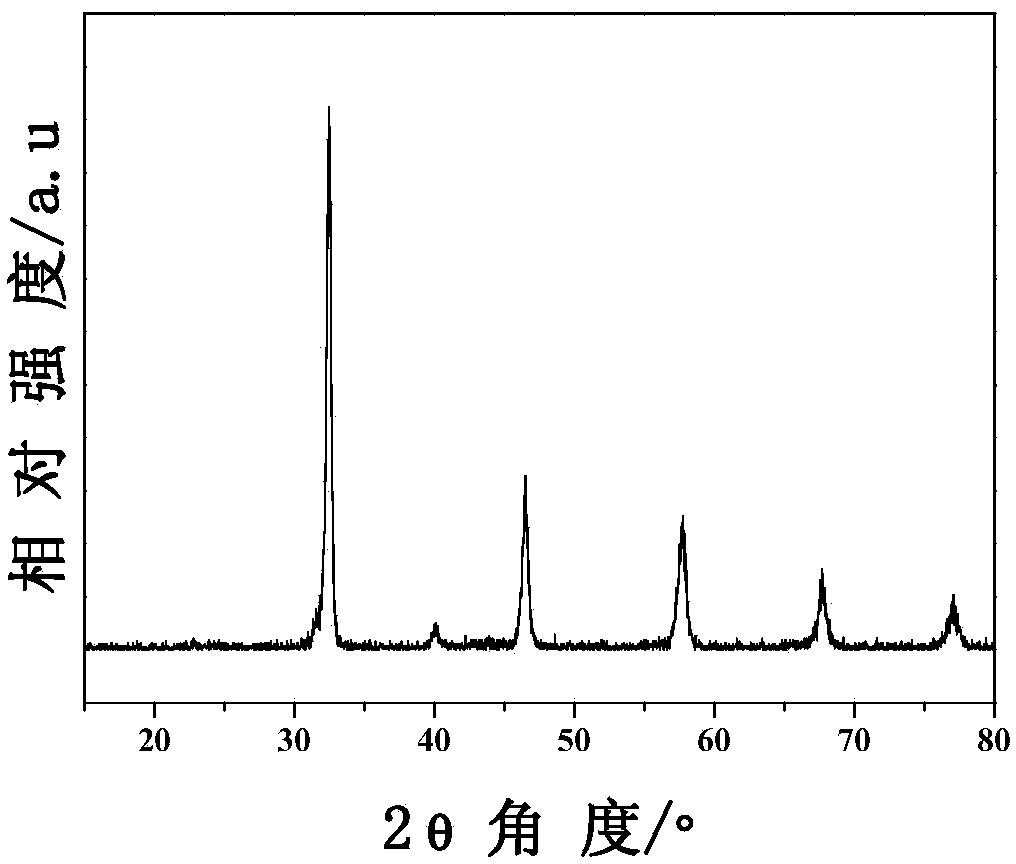
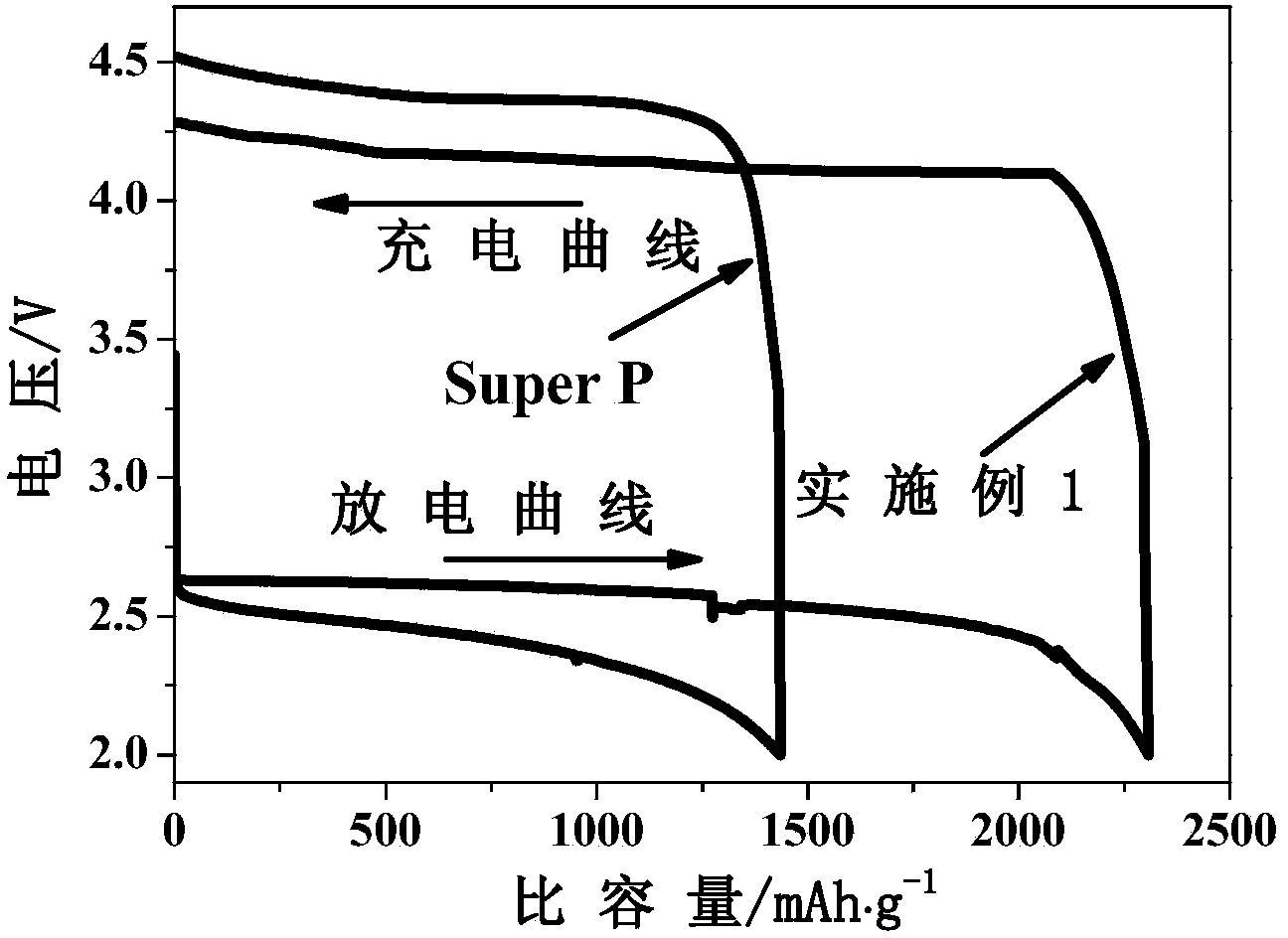
[0030] Weigh 4.23g Sr(NO 3 ) 2 , 1.77g (NH 4 )6Mo 7 o 24 4H 2 O and 4.00g Cr(NO 3 ) 3 9H 2 O was dissolved in 100ml of deionized water; 12.61g of citric acid and 11.69g of ethylenediaminetetraacetic acid were added; then evaporated and concentrated at 65°C for 12h to obtain a gel; the obtained gel was dried at 250°C for 6h in an air atmosphere to obtain a xerogel; Grind the xerogel into a powder, and bake it at 800°C for 6 hours in an air atmosphere; the obtained powder is pressed at 300 MPa for 5 minutes; finally, it is heated in 5% H 2 / 95% Ar atmosphere and calcined at 1200°C for 24h to prepare the catalyst. In conjunction with the literature Journal of Solid State Chemistry 155 (2000) 233-237, it can be judged that the obtained material is a pure-phase double perovskite structure (attached figure 1 ). attached by figure 2 It can be seen from the scanning electron microscope image of the obtained material that the material ...
Embodiment 2
[0031] Example 2: Ca 2 CrMoO 6
[0032] Weigh 4.72g Ca㈩O 3 ) 2 4H 2 O, 1.77g (NH 4 ) 6 Mo 7 o 24 4H 2 O and 4.00gCr(NO 3 ) 3 9H 2 O was dissolved in 100ml of deionized water; 12.61g of citric acid and 1.69g of ethylenediaminetetraacetic acid were added; then evaporated and concentrated at 65°C for 4h to obtain a gel; the obtained gel was dried at 200°C for 12h in an air atmosphere to obtain a xerogel The obtained xerogel was ground into powder and calcined at 500°C for 12h in an air atmosphere; the obtained powder was pressed into tablets at 300MPa for 15min; finally, the catalyst was obtained by calcining at 1200°C for 24h in an atmosphere of 15% H2 / 85% Ar.
Embodiment 3
[0033] Example 3: Ba 2 CrMoO 6
[0034] Weigh 5.23g Ba(NO 3 ) 2 , 1.77g (NH 4 ) 6 Mo 7 o 24 4H 2 O and 4.00g Cr㈩O 3 ) 3 9H 2 O was dissolved in 100ml of deionized water; 12.61g of citric acid and 11.69g of ethylenediaminetetraacetic acid were added; then evaporated and concentrated at 65°C for 1h to obtain a gel; the obtained gel was dried in an air atmosphere at 250°C for 6h to obtain a xerogel; The xerogel was ground into powder and calcined at 1000°C for 1 h in an air atmosphere; the obtained powder was pressed into tablets at 300 MPa for 20 min; and finally calcined at 800°C for 12 h in a 30% H2 / 70% Ar atmosphere to obtain a catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com