Double-perovskite type intermediate temperature solid oxide fuel cell anode material and preparation method
A fuel cell cathode, solid oxide technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problem of high thermal expansion coefficient, and achieve the effects of low thermal expansion coefficient, high electronic conductivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
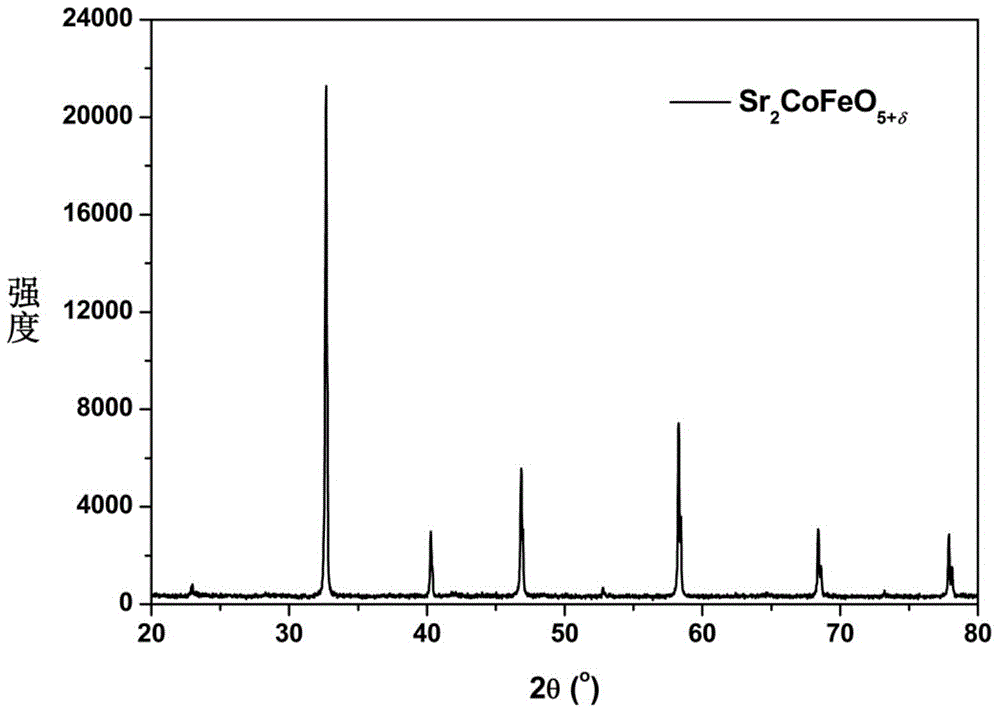
[0032] Cathodic material Sr for intermediate temperature solid oxidation fuel cells with double perovskite structure prepared by wet chemical method 2 CoFeO 5+δ . The preparation method is as follows:
[0033] 1) Using analytically pure Sr(NO 3 ) 2 , Co(NO 3 ) 2 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O is the raw material, and the required experimental raw materials are weighed according to the stoichiometric ratio.
[0034] 2) With anhydrous ethylene glycol and citric acid (the molar ratio of the two is 1: 1) as complexing agent, take the complexing agent whose molar number is 1~3 times of the sum of all metal cations in the sample, and step 1 The weighed experimental raw materials were dissolved in deionized water together.
[0035] 3) Place the beaker containing the solution in step 2 on a magnetic stirrer and heat and stir until a gel is formed.
[0036] 4) Dry the gel sample obtained in step 3 in an oven at a temperature of 150-300° C. for 2-5 hours to form a xerogel.
[...
Embodiment 2
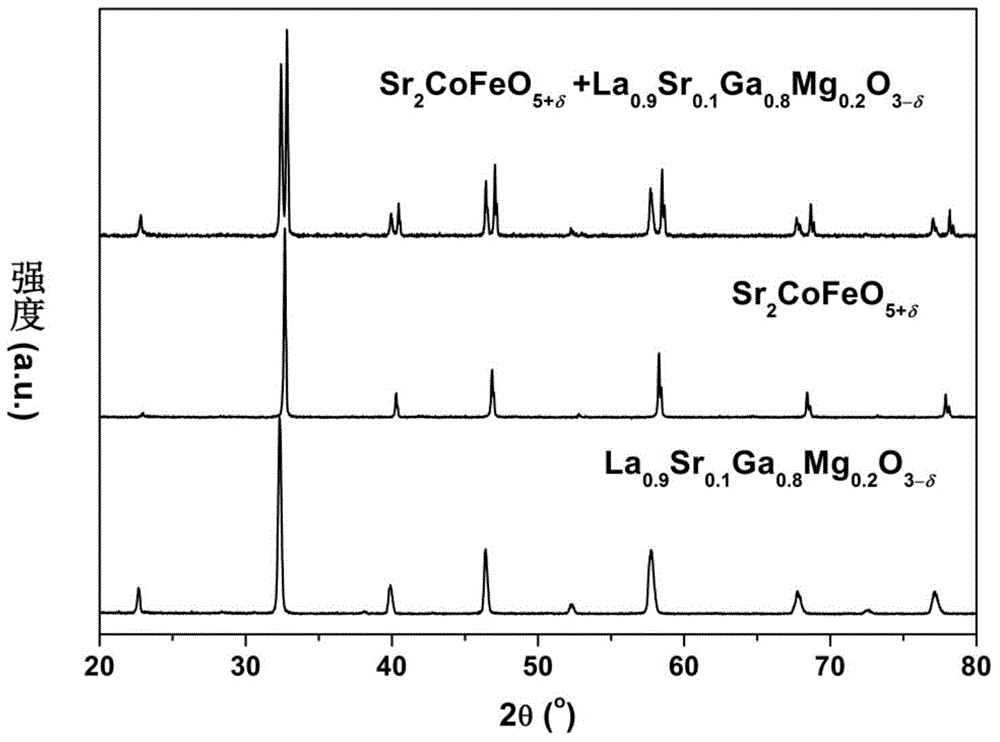
[0042] The raw material Sr(NO in embodiment 1 3 ) 2 replaced by Ca(NO 3 ) 2 , the rest of the raw materials and complexing agent remain unchanged, and Ca 2 CoFeO 5+δ cathode material. Ca 2 CoFeO 5+δ with La 0.9 Sr 0.1 Ga 0.8 Mg 0.2 o 3-δ Electrolyte materials also have a good chemical match.
Embodiment 3
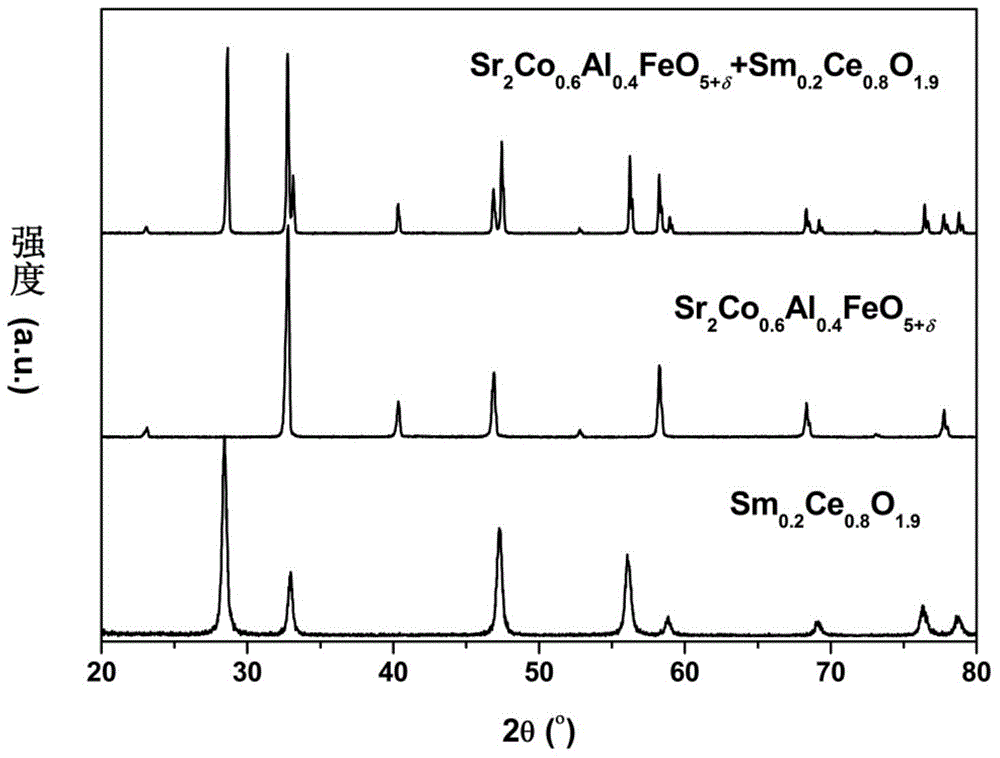
[0044] The raw material Sr(NO in embodiment 1 3 ) 2 Replaced by Ba(NO 3 ) 2 , the rest of the raw materials and complexing agent remain unchanged, and Ba 2 CoFeO 5+δ cathode material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com