Double-perovskite composite metal oxide catalyst and preparation method and application thereof
A composite metal and double perovskite technology, applied in the field of air pollution control, can solve the problems of catalyst sintering and deactivation, and achieve the effect of simple synthesis method, low price and good industrial application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] First, weigh lanthanum nitrate, copper acetate, and nickel nitrate as precursors at a molar ratio of 2:1:1, dissolve them in 10 mL of deionized water at room temperature, and stir the solution to a clear state; ratio) = 1.5, add citric acid to the above solution, stir the solution to a clear state; then, place the resulting solution in a water bath at 70°C, and magnetically stir until the solution is in a gel state; then, bake the gel at 100°C Dry for 24 hours; finally, the obtained solid is calcined at 500°C for 4h, at 1100°C for 4h, and then lowered to room temperature. The obtained catalyst is denoted as La 2 CuNiO 6 .
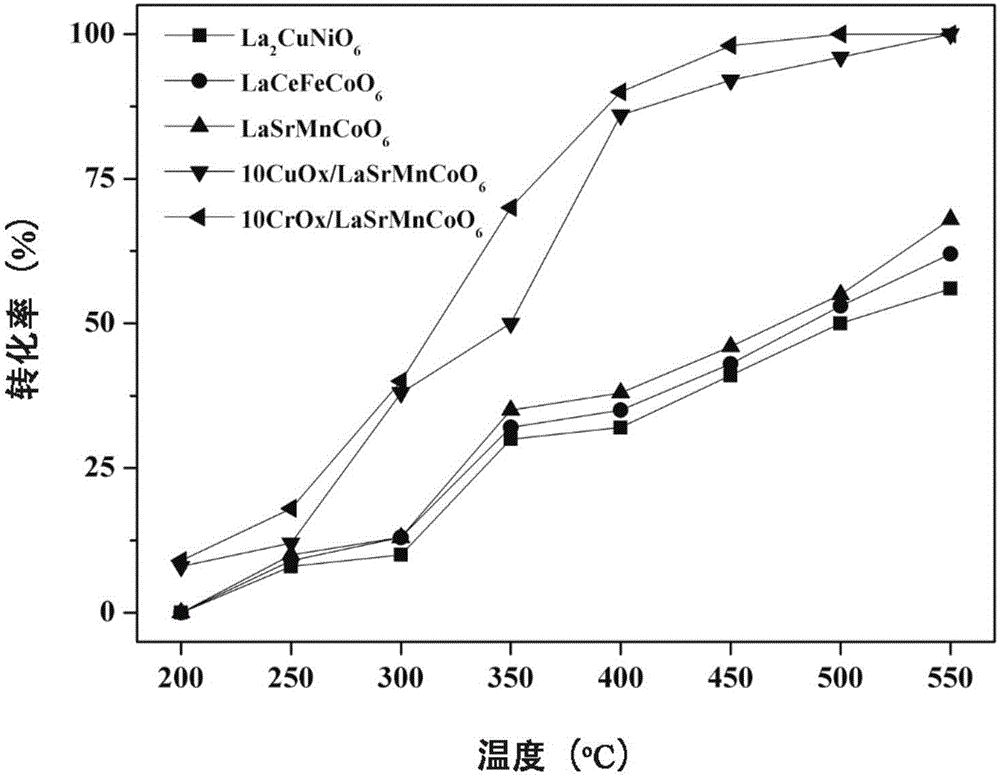
[0028] The catalytic reaction is carried out in a fixed bed, and 0.5g of La 2 CuNiO 6 , with 1,2-dichloroethane (DCE) as the probe gas, the concentration of the reactant is controlled at 400ppm, and the reaction space velocity is 24000h -1 , the oxygen concentration was 20%, the reaction was maintained at each temperature point for 40 min, and th...
Embodiment 2
[0030] First, weigh lanthanum nitrate, cerium nitrate, iron nitrate and cobalt nitrate as precursors at a molar ratio of 1:1:1:1, dissolve them in 10 mL of deionized water at room temperature, and stir the solution until it is clear; then, according to citric acid: total The number of metal ions (molar ratio) = 1.5, citric acid was added to the above solution, and the solution was stirred to a clear state; after that, the resulting solution was placed in a water bath at 70°C, and magnetically stirred until the solution was in a gel state; then, the gel Dry at 100°C for 24h; finally, roast the obtained solid at 500°C for 4h, then at 1100°C for 4h, and drop to room temperature. The obtained catalyst is recorded as LaCeFeCoO6 ;
[0031] The catalytic reaction is carried out in a fixed bed, take 0.5g of LaCeFeCoO 6 , also using DCE as the probe gas, the reaction conditions are the same as in Example 1, and the experimental results show that at 200°C, 250°C, 300°C, 350°C, 400°C, 45...
Embodiment 3
[0033] First, weigh lanthanum nitrate, strontium nitrate, manganese acetate, and cobalt nitrate as precursors at a molar ratio of 1:1:1:1, dissolve them in 10 mL of deionized water at room temperature, and stir the solution to a clear state; then, according to citric acid: total The number of metal ions (molar ratio) = 1.5, citric acid was added to the above solution, and the solution was stirred to a clear state; after that, the resulting solution was placed in a water bath at 70°C, and magnetically stirred until the solution was in a gel state; then, the gel Dry at 100°C for 24h; finally, roast the obtained solid at 550°C for 4h, then at 1100°C for 4h, and lower it to room temperature. The obtained catalyst is recorded as LaSrMnCoO 6 ;
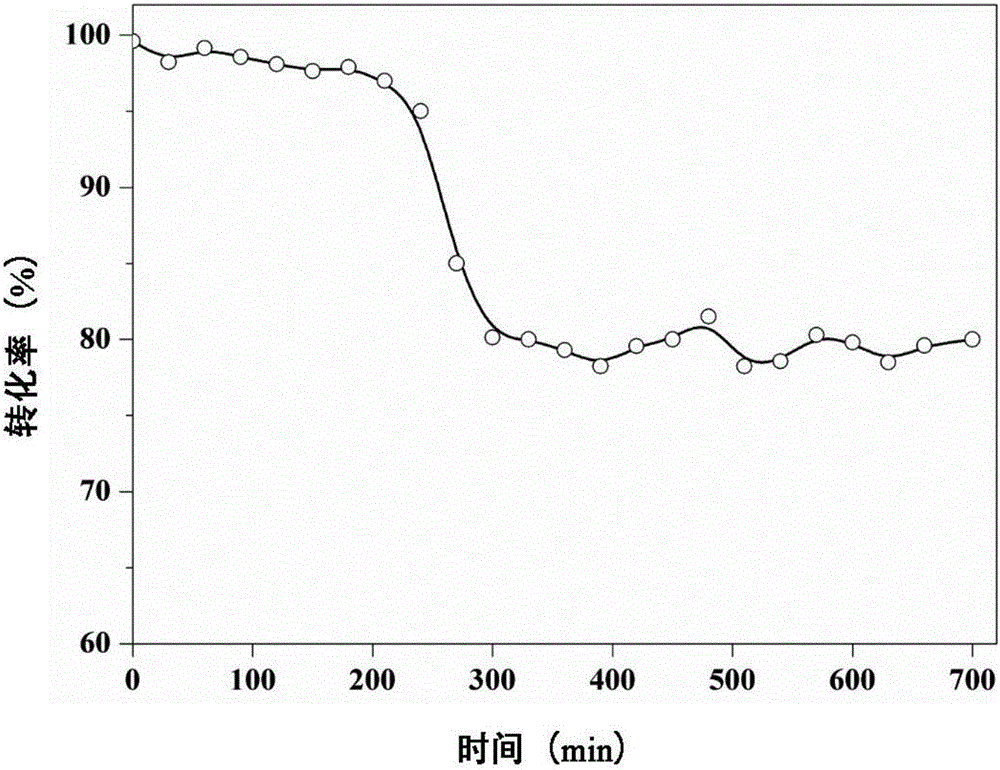
[0034] The catalytic reaction is carried out in a fixed bed, take 0.5g of LaSrMnCoO 6 , also using DCE as the probe gas, the reaction conditions are the same as in Example 1, and the experimental results show that at 200°C, 250°C, 300°C, 35...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com