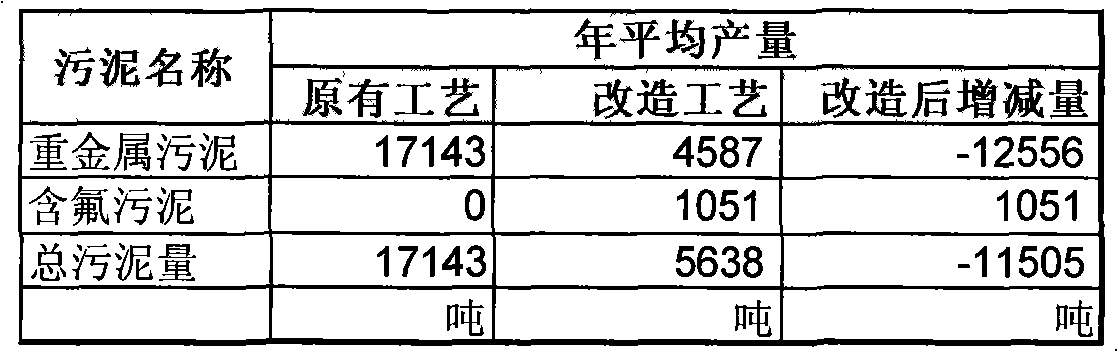
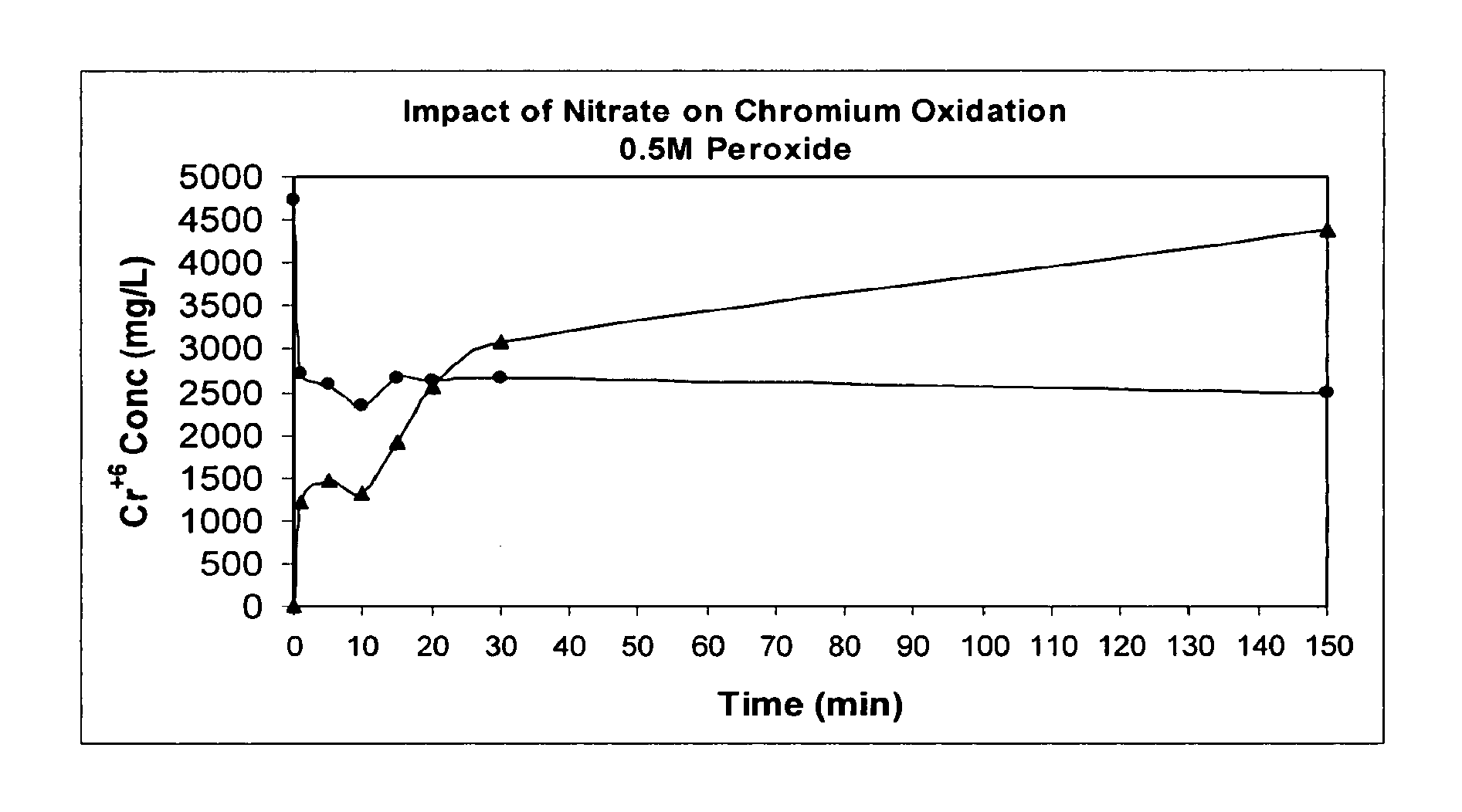
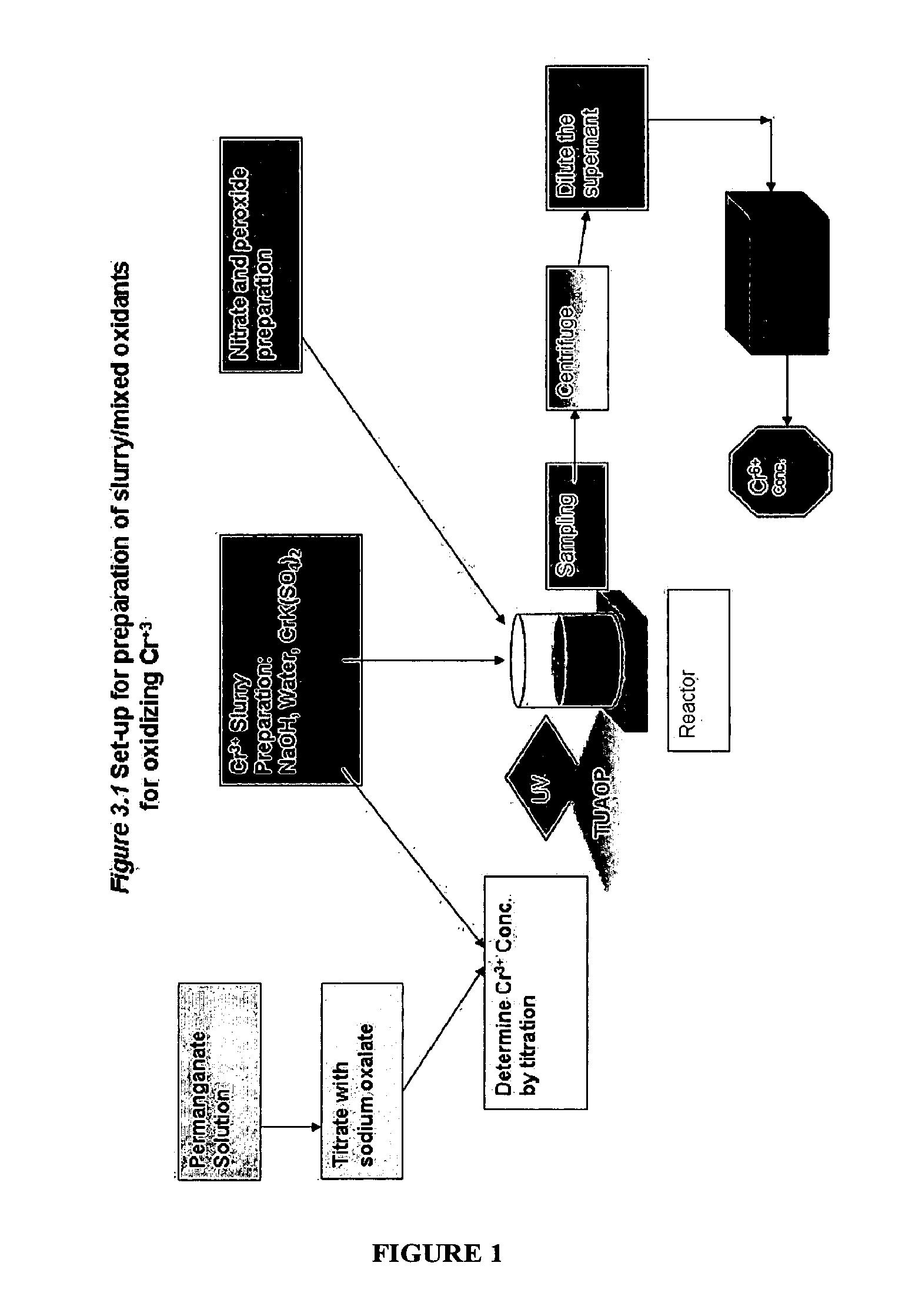
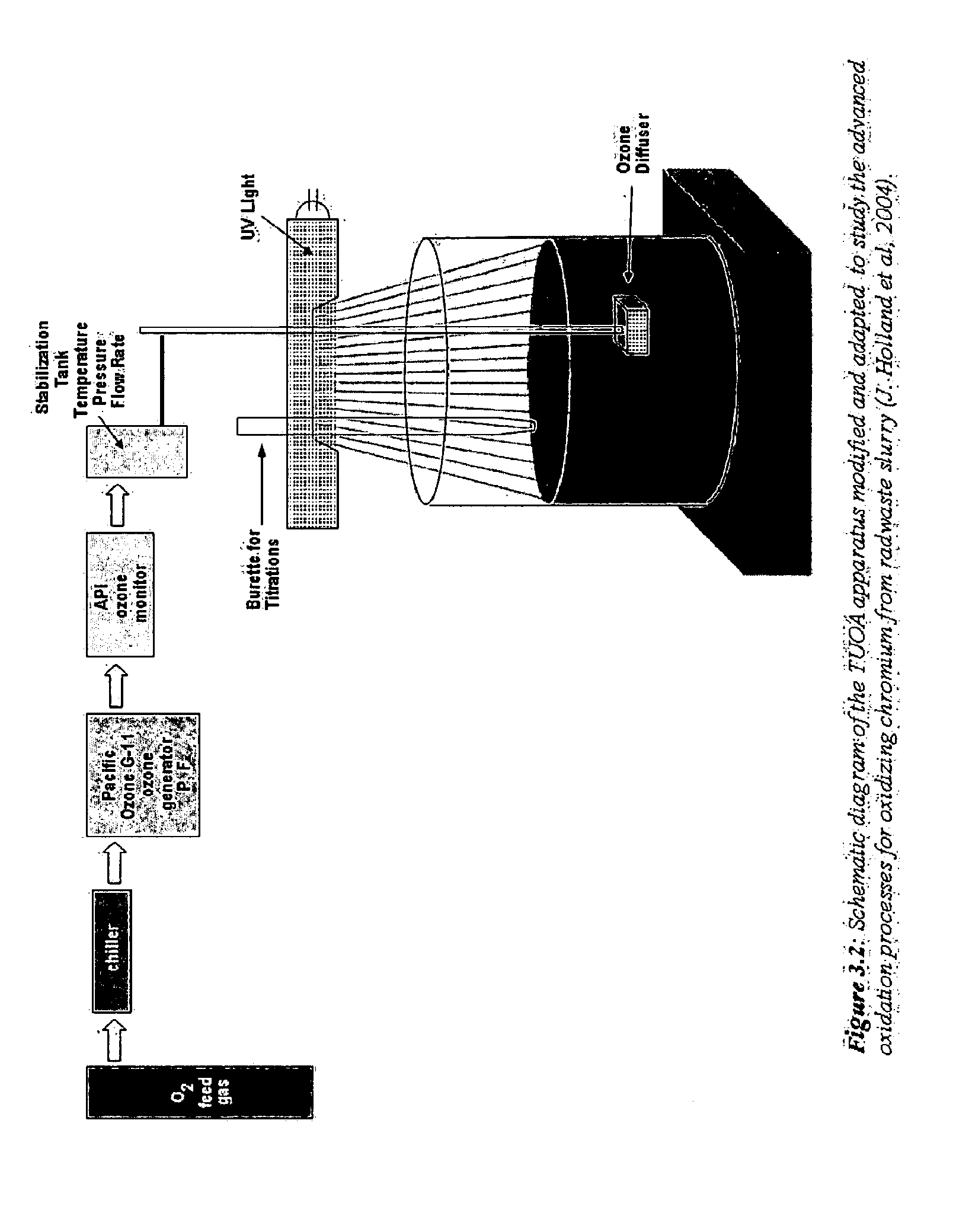
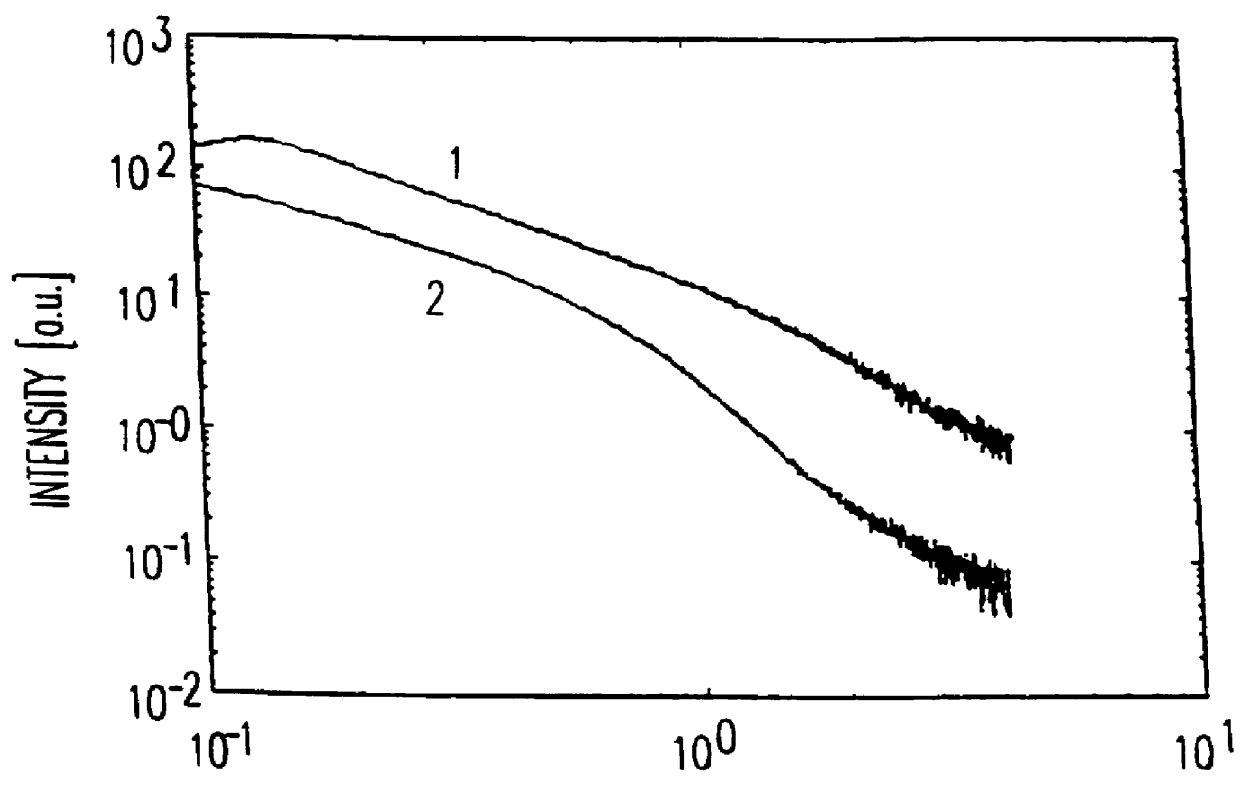
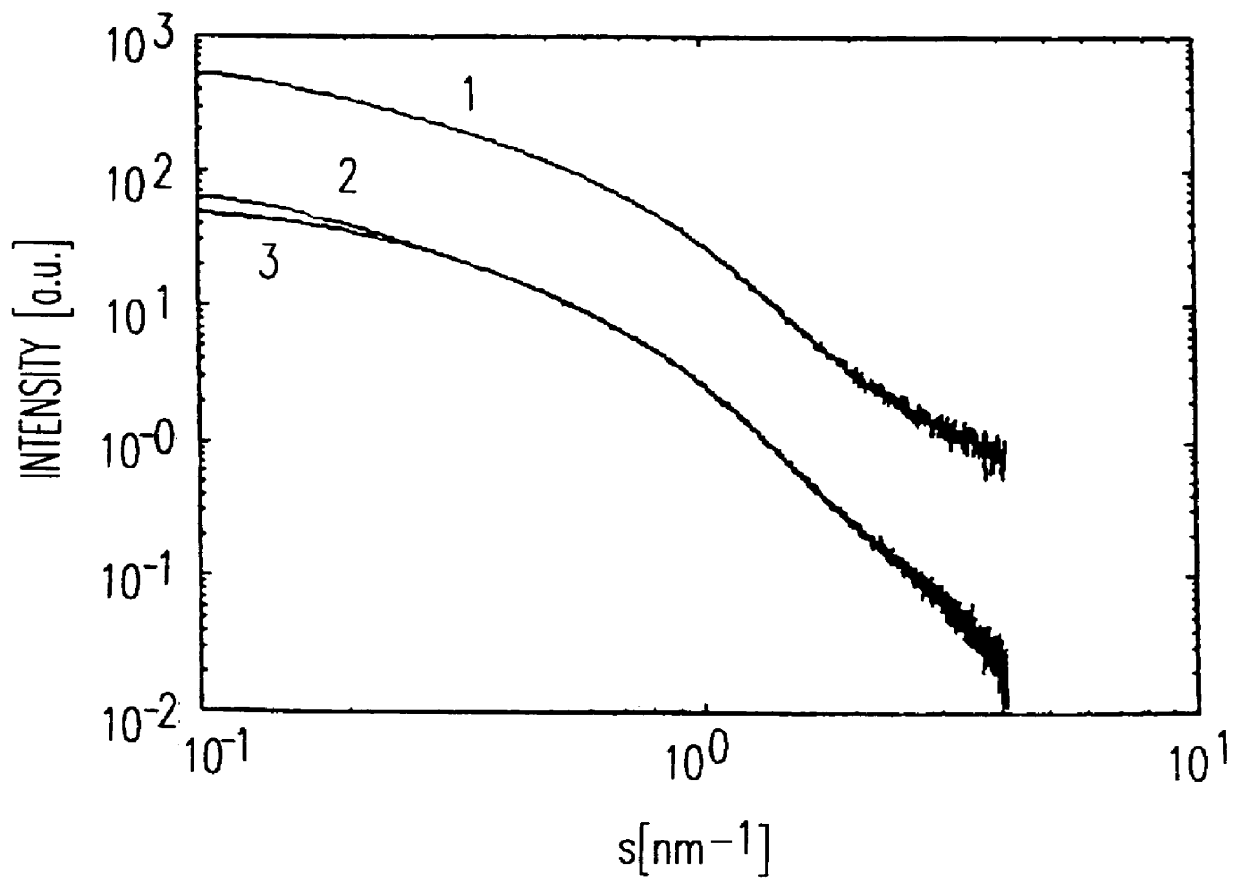
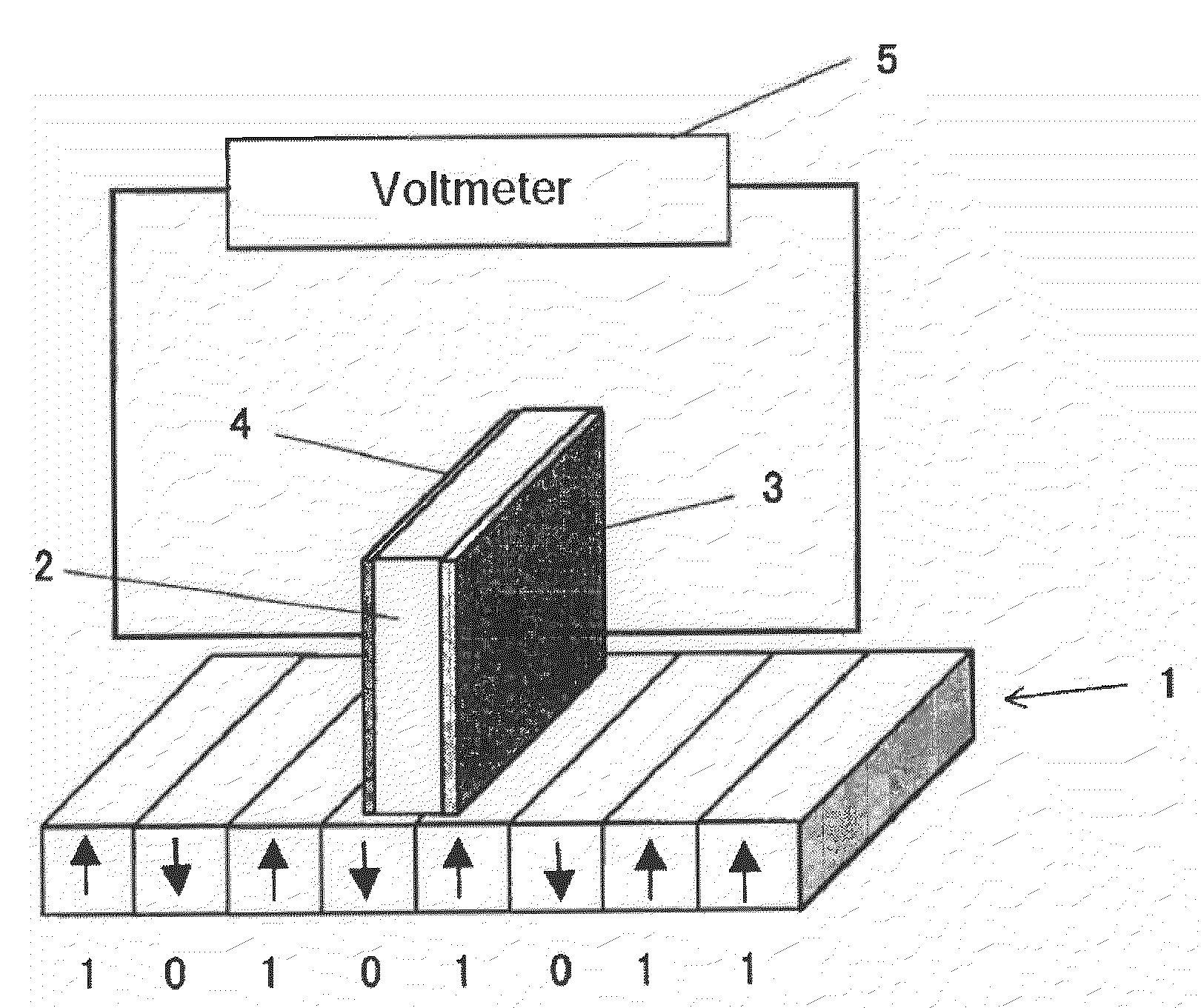
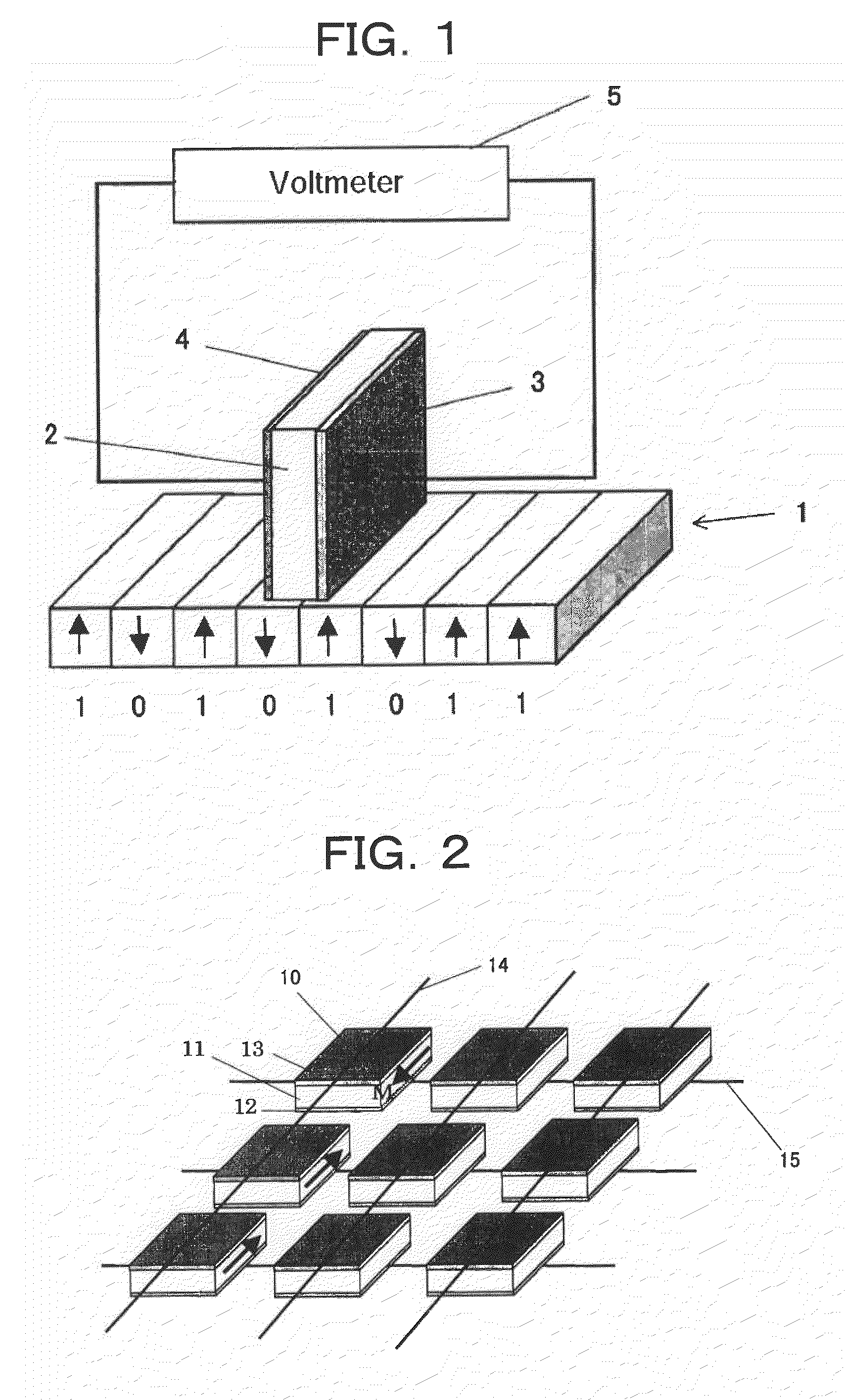
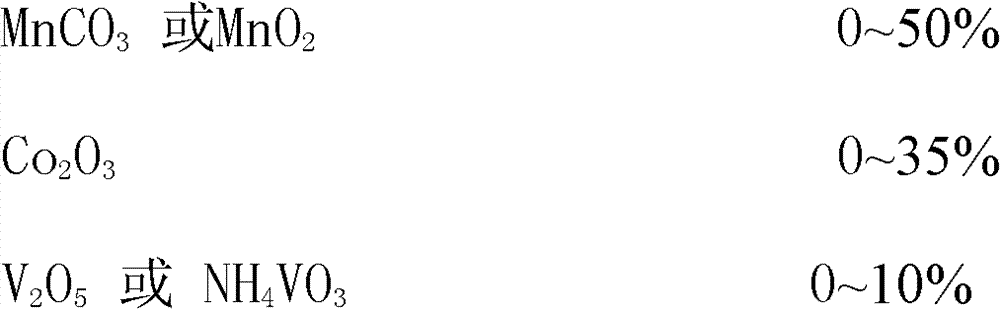Patents
Literature
281results about "Chromium oxides/hydrates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
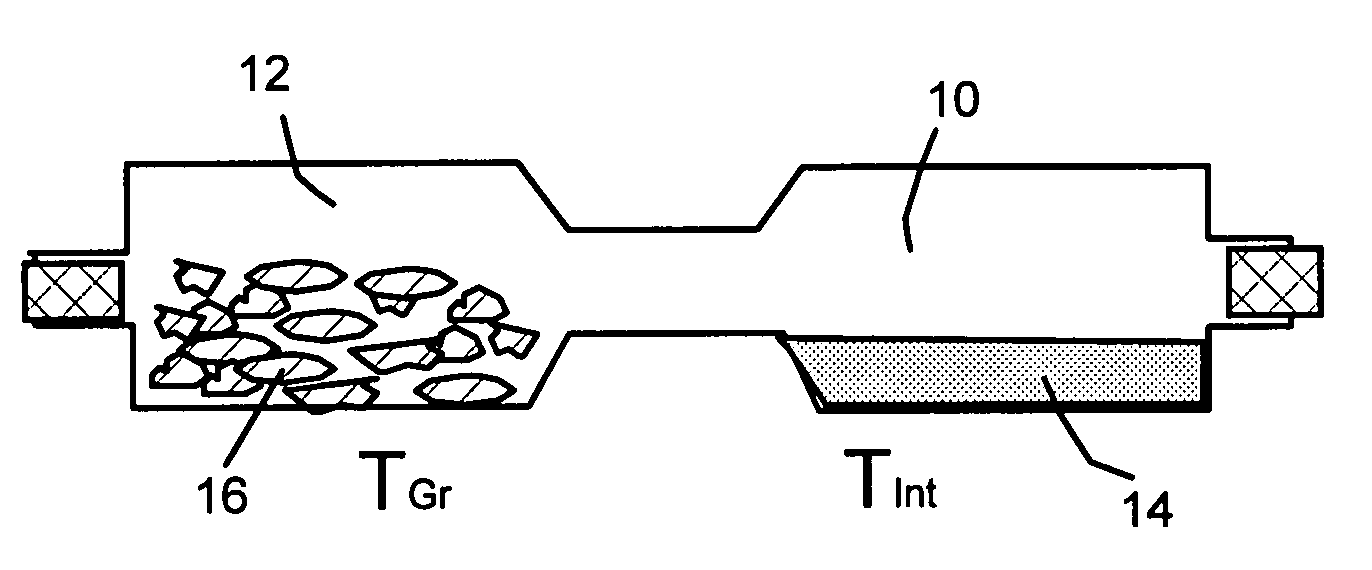
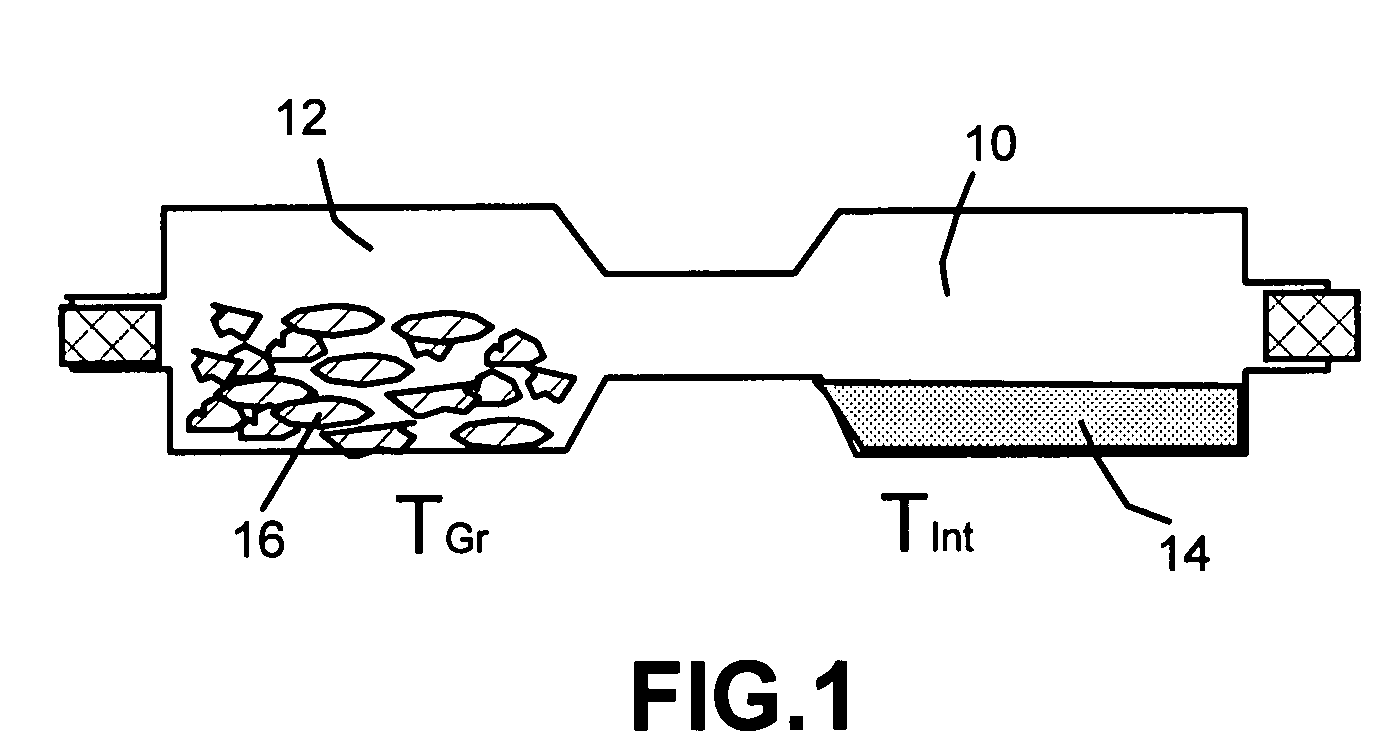
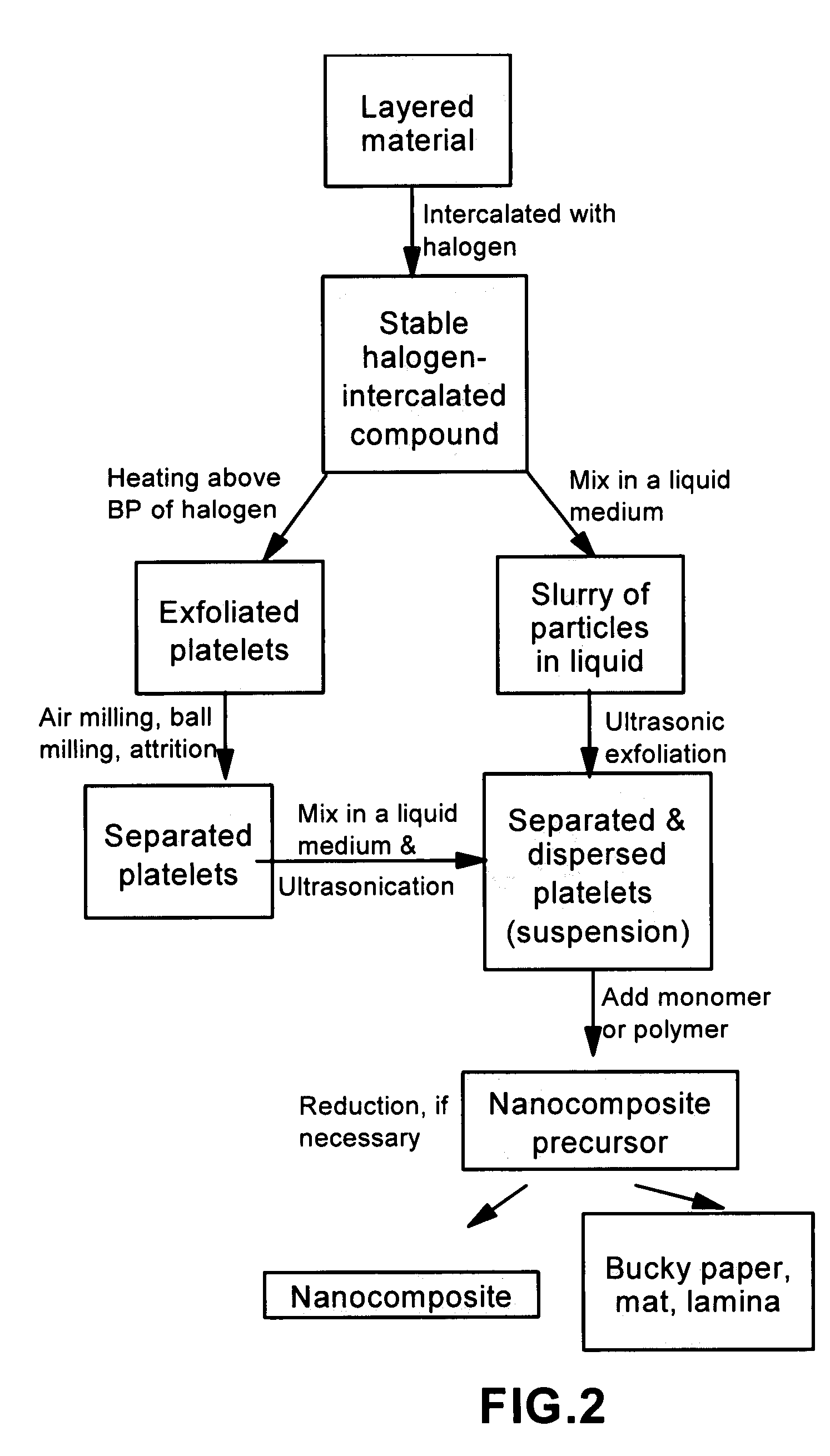
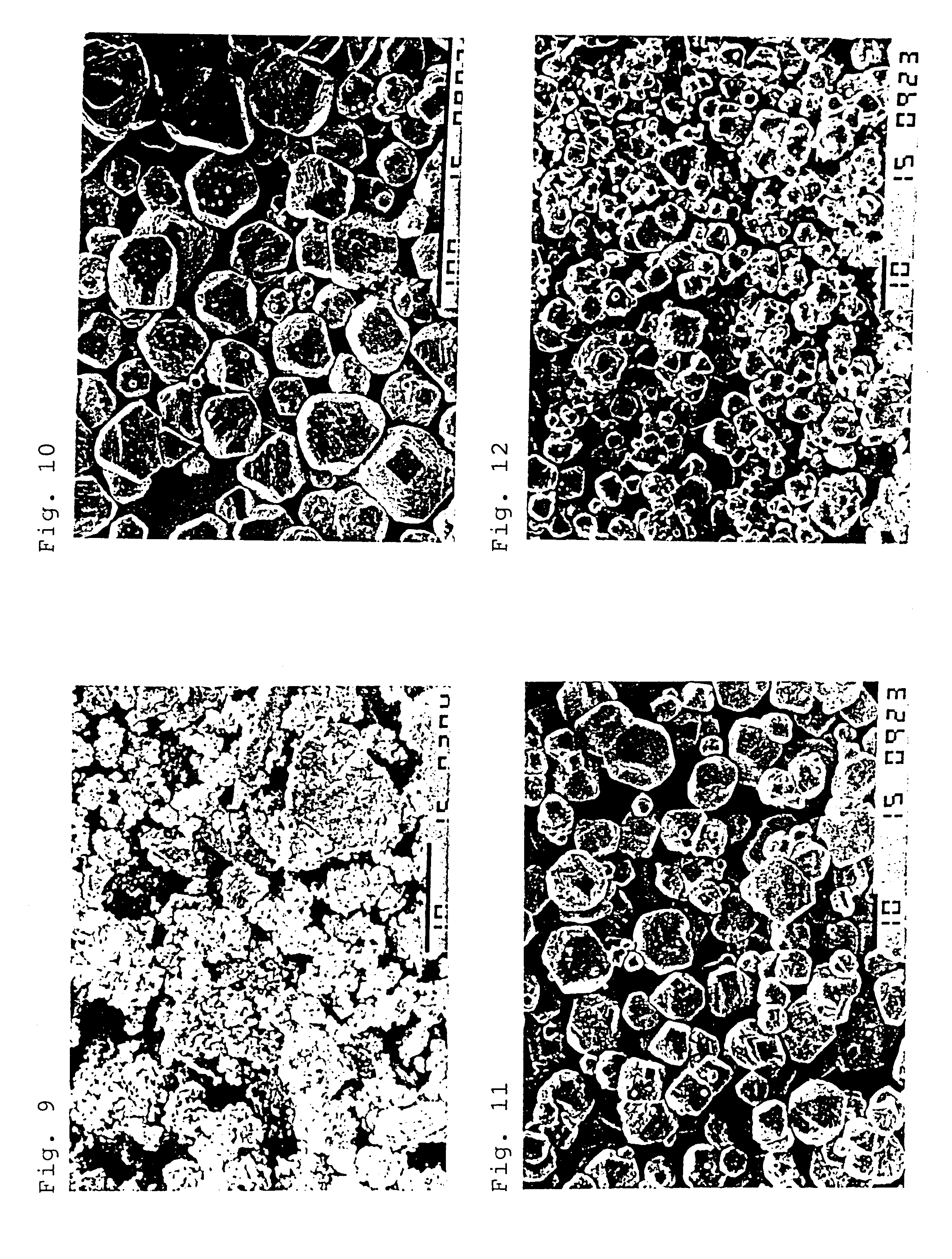
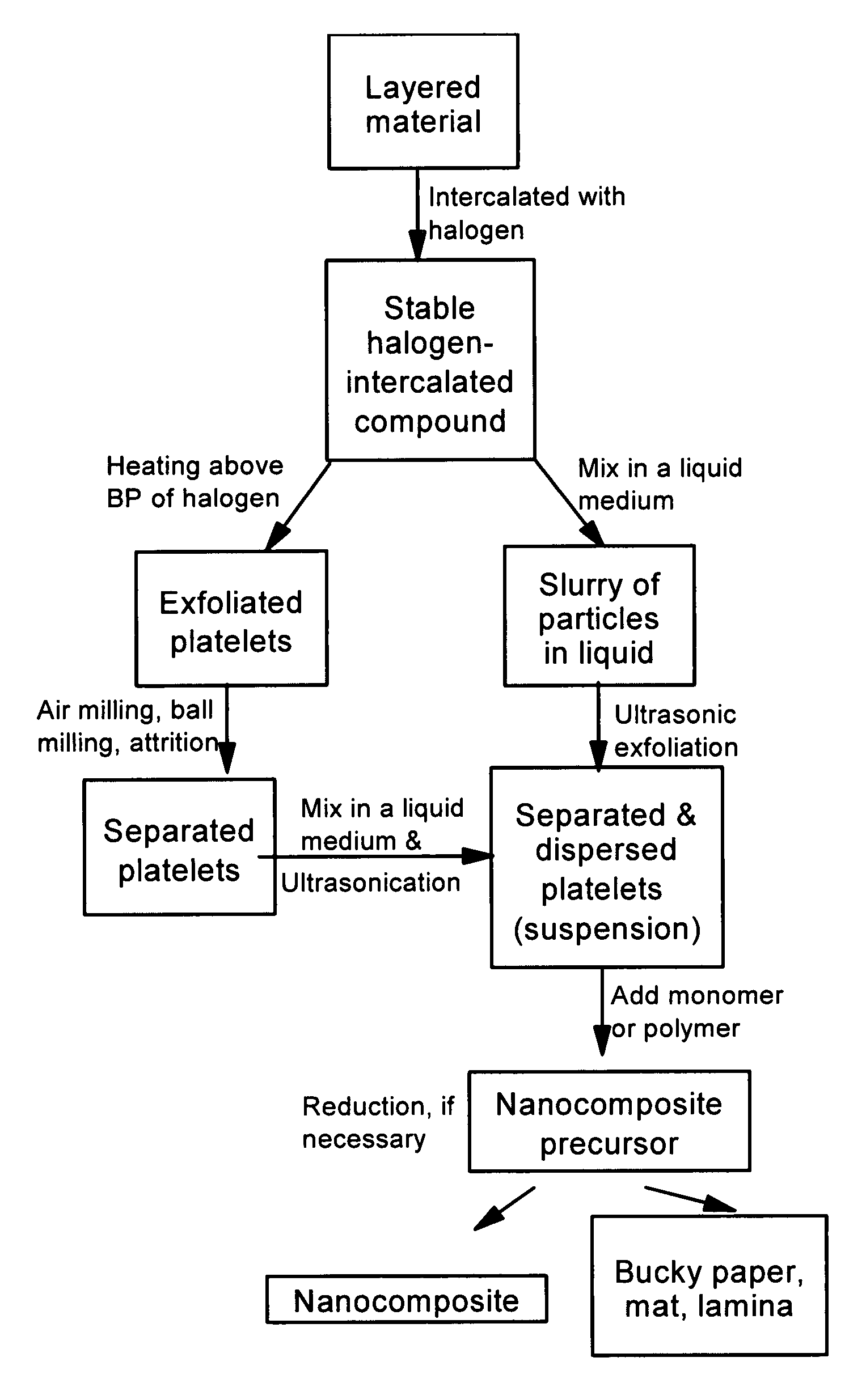
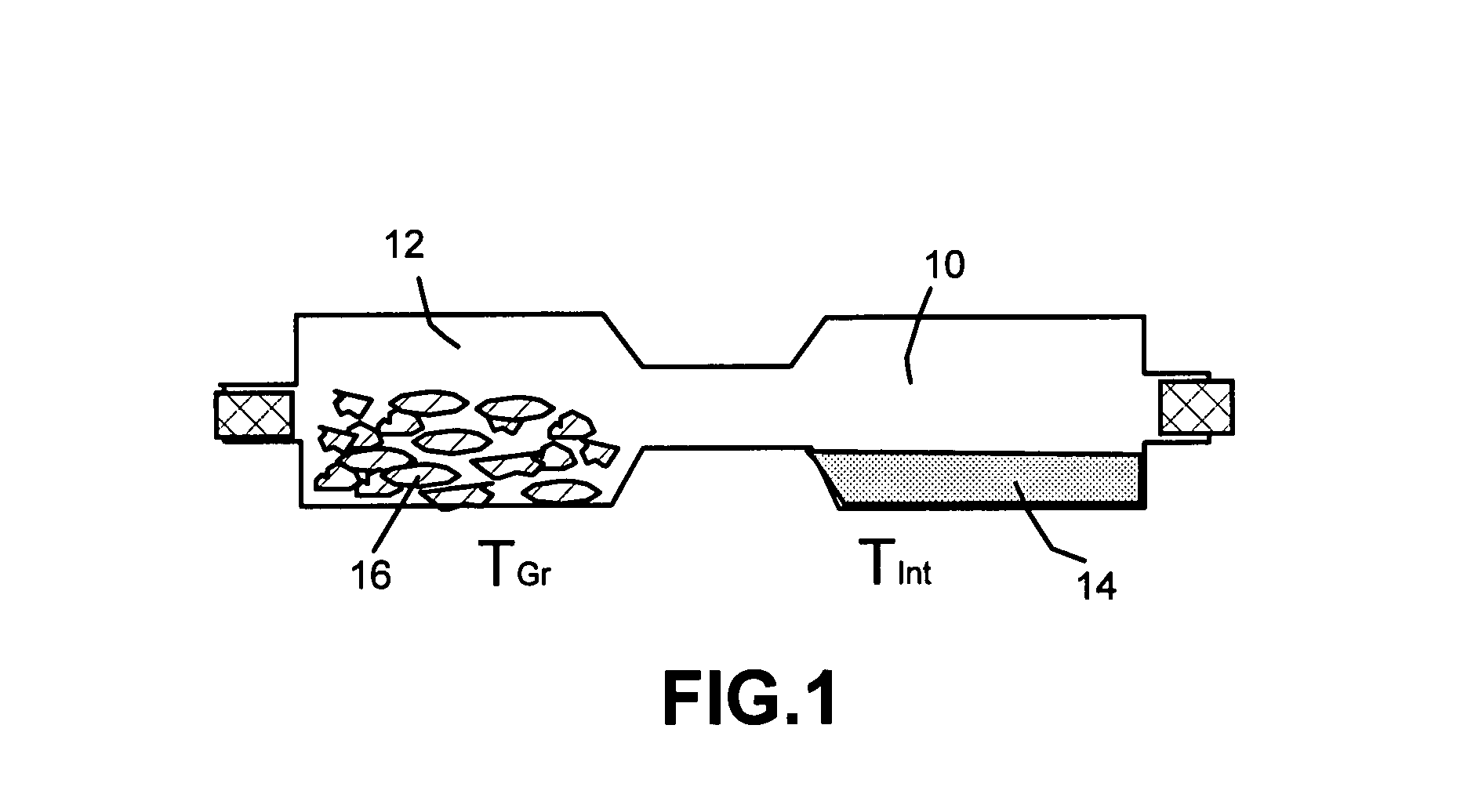
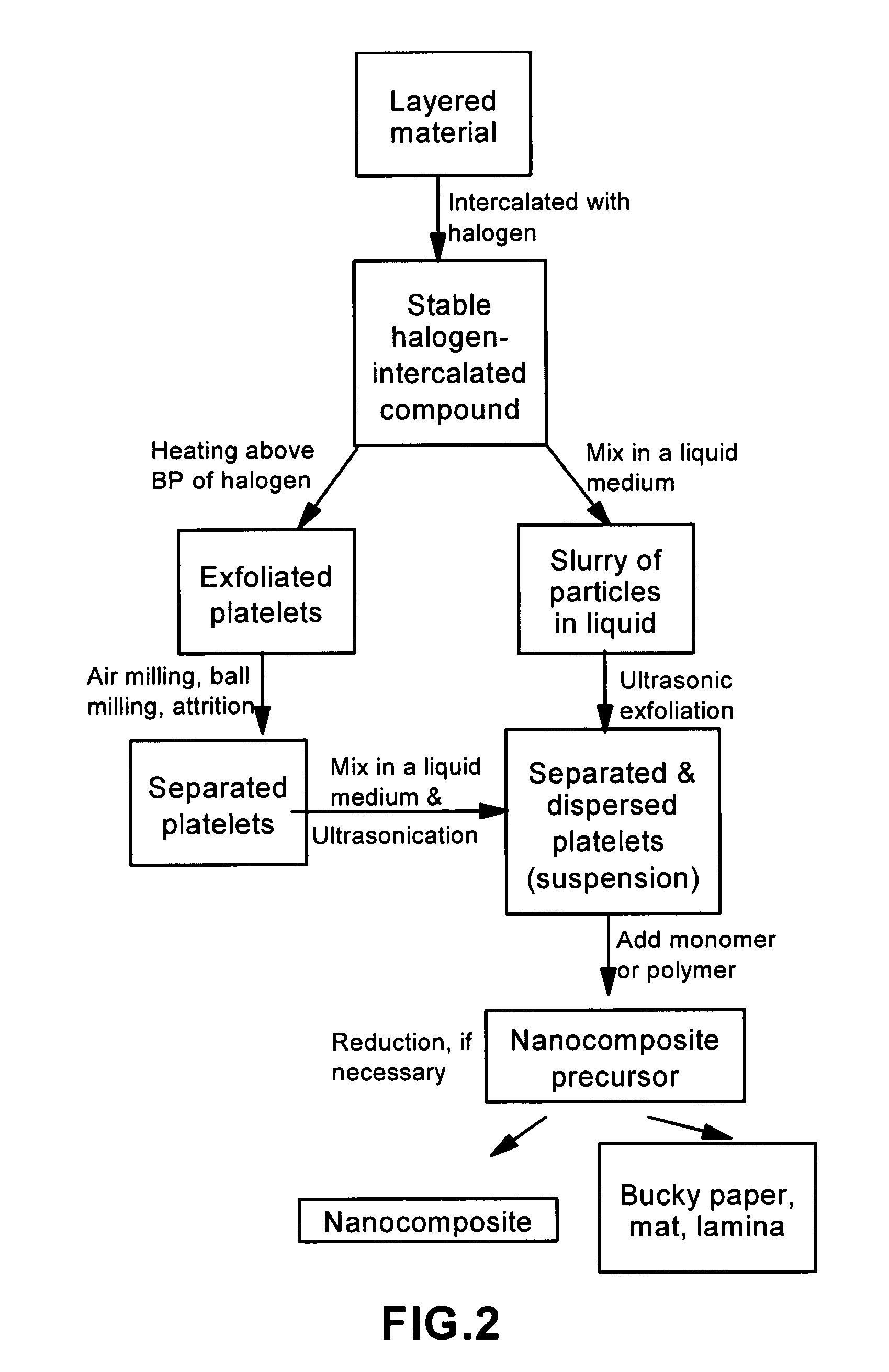
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS20080206124A1Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumPhysical chemistry
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
Process for the production of ultrafine particles
A new, cost effective process for the production of ultrafine particles which is based on mechanically activated chemical reaction of a metal compound with a suitable reagent. The process involves subjecting a mixture of a metal compound and a suitable reagent to mechanical activation to increase the chemical reactivity of the reactants and / or reaction kinetics such that a chemical reaction can occur which produces a solid nano-phase substance. Concomitantly, a by-product phase is also formed. This by-product phase is removed so that the solid nano-phase substance is left behind in the form of ultrafine particles. During mechanical activation a composite structure is formed which consists of an intimate mixture of nano-sized grains of the nano-phase substance and the reaction by-product phase. The step of removing the by-product phase, following mechanical activation, may involve subjecting the composite structure to a suitable solvent which dissolves the by-product phase, while not reacting with the solid nano-phase substance. The process according to the invention may be used to form ultrafine metal powders as well as ultrafine ceramic powders. Advantages of the process include a significant degree of control over the size and size distribution of the ultrafine particles, and over the nature of interfaces created between the solid nano-phase substance and the reaction by-product phase.
Owner:WESTERN AUSTRALIA UNIV OF THE
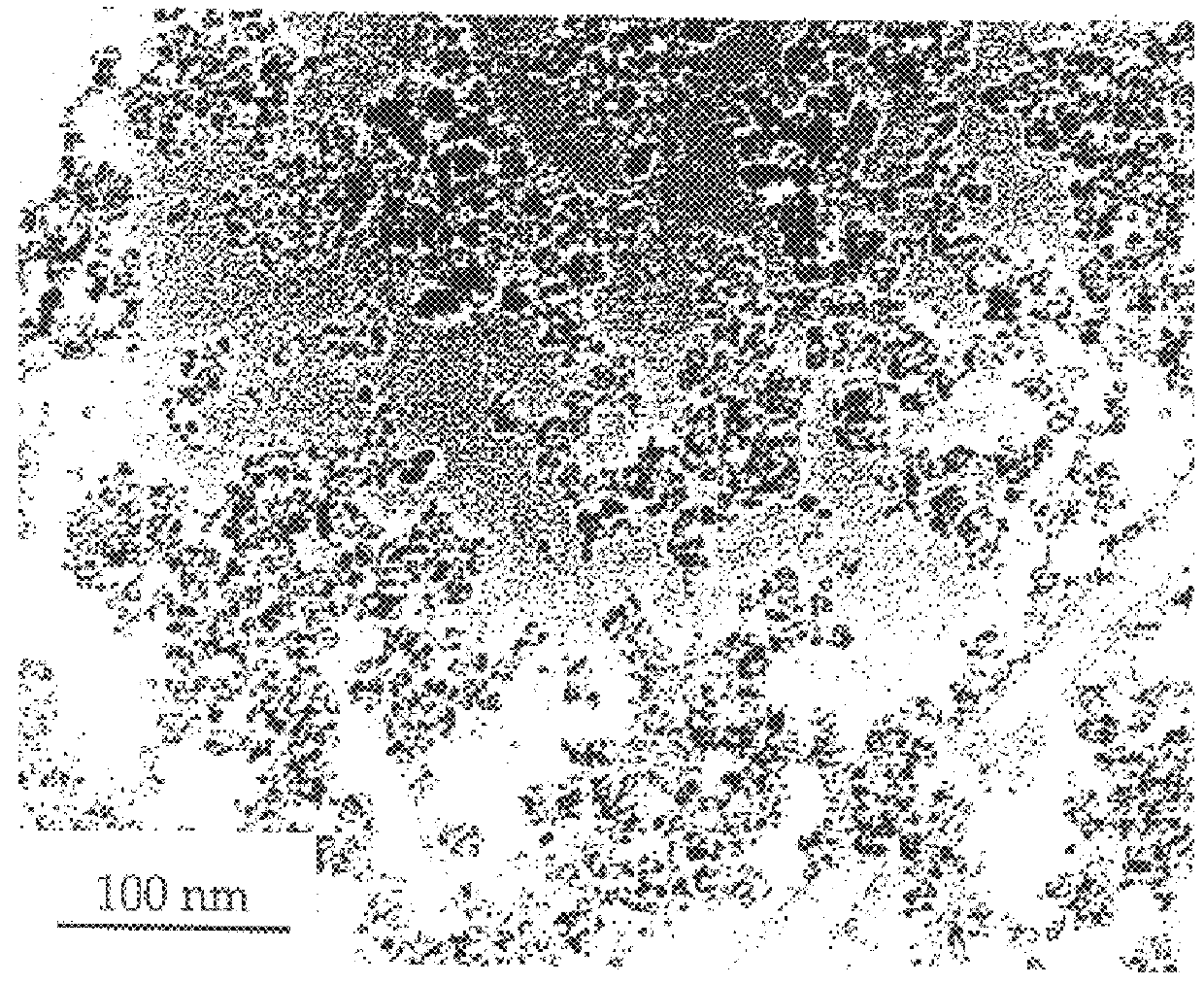
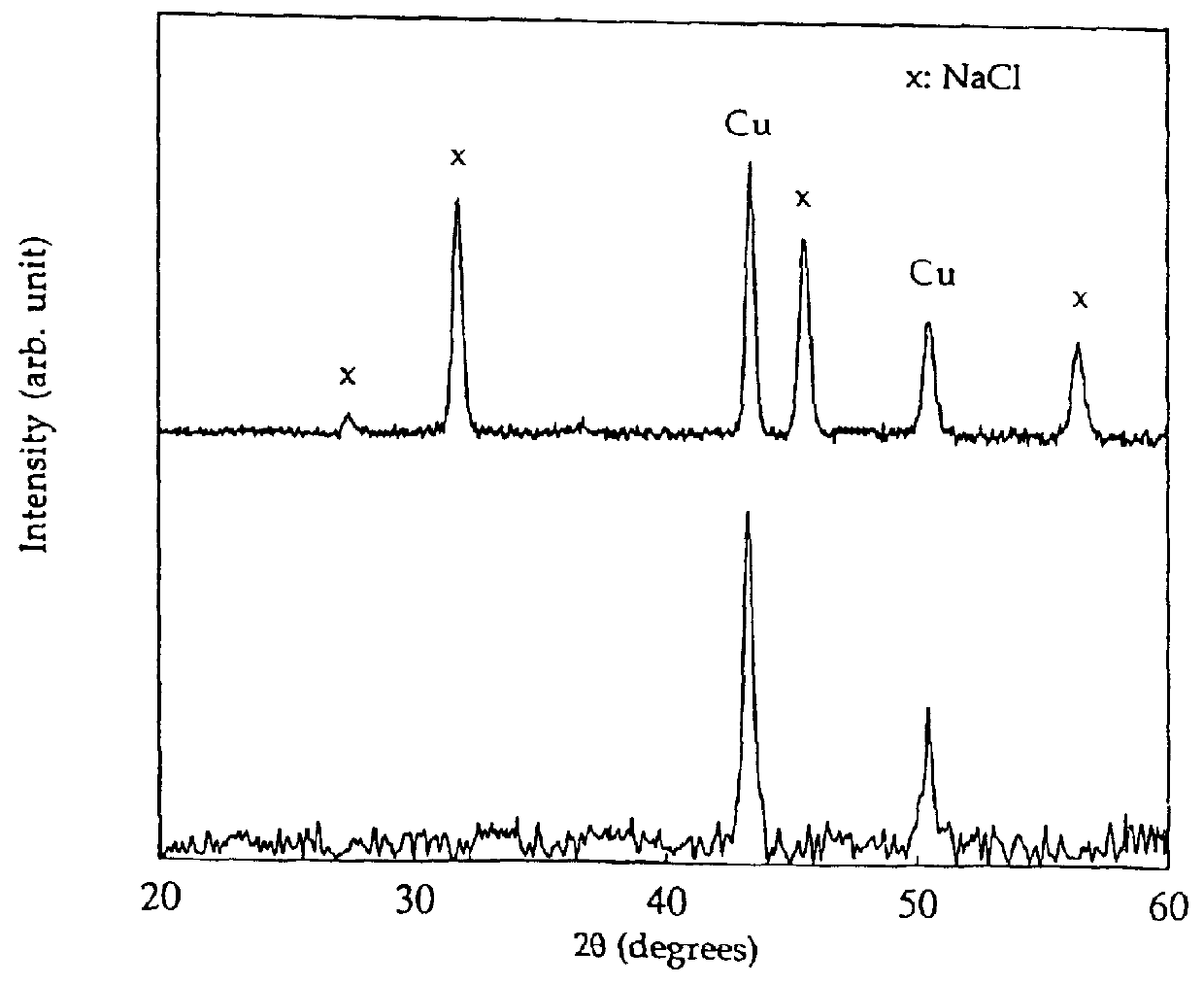
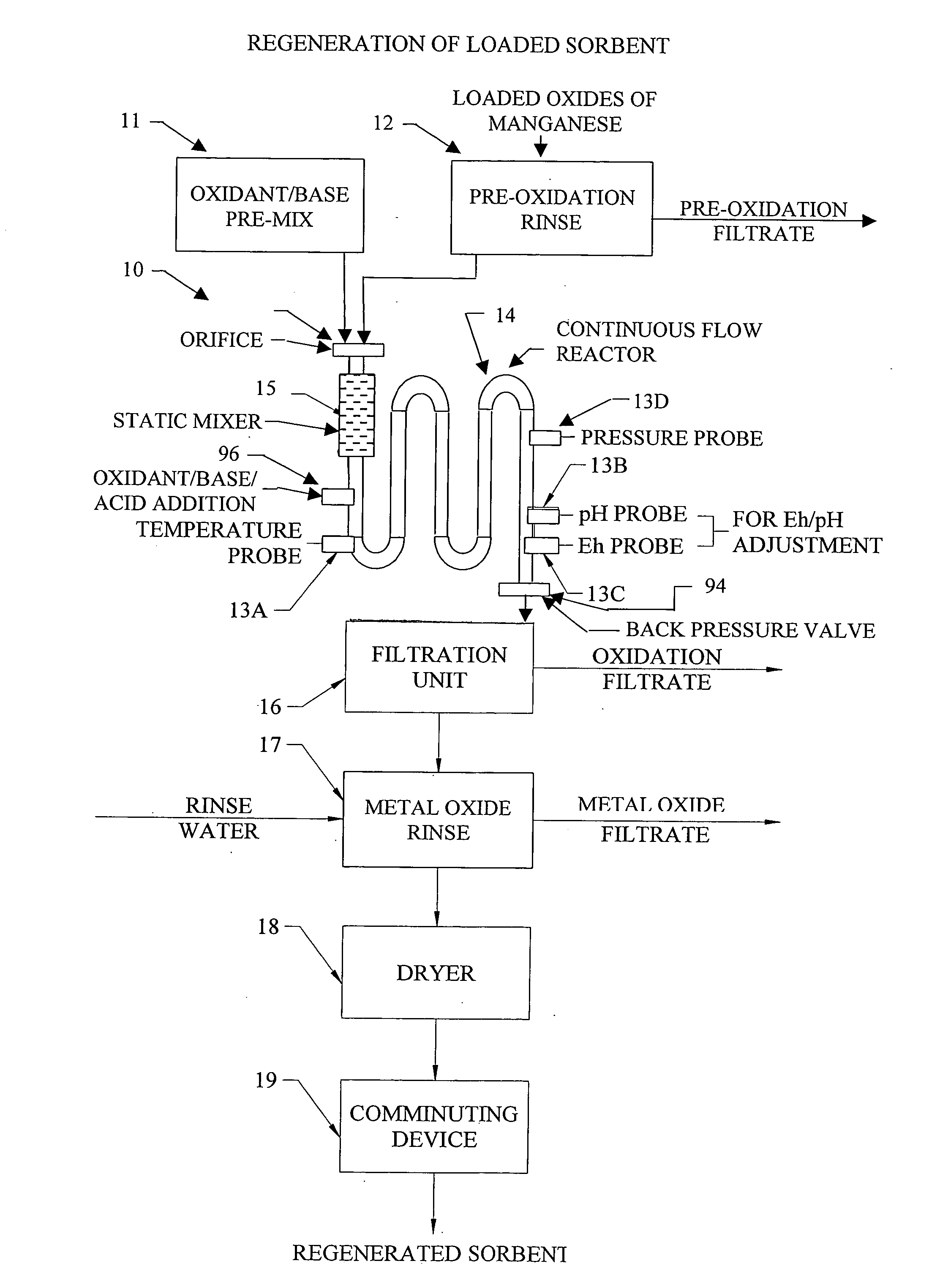
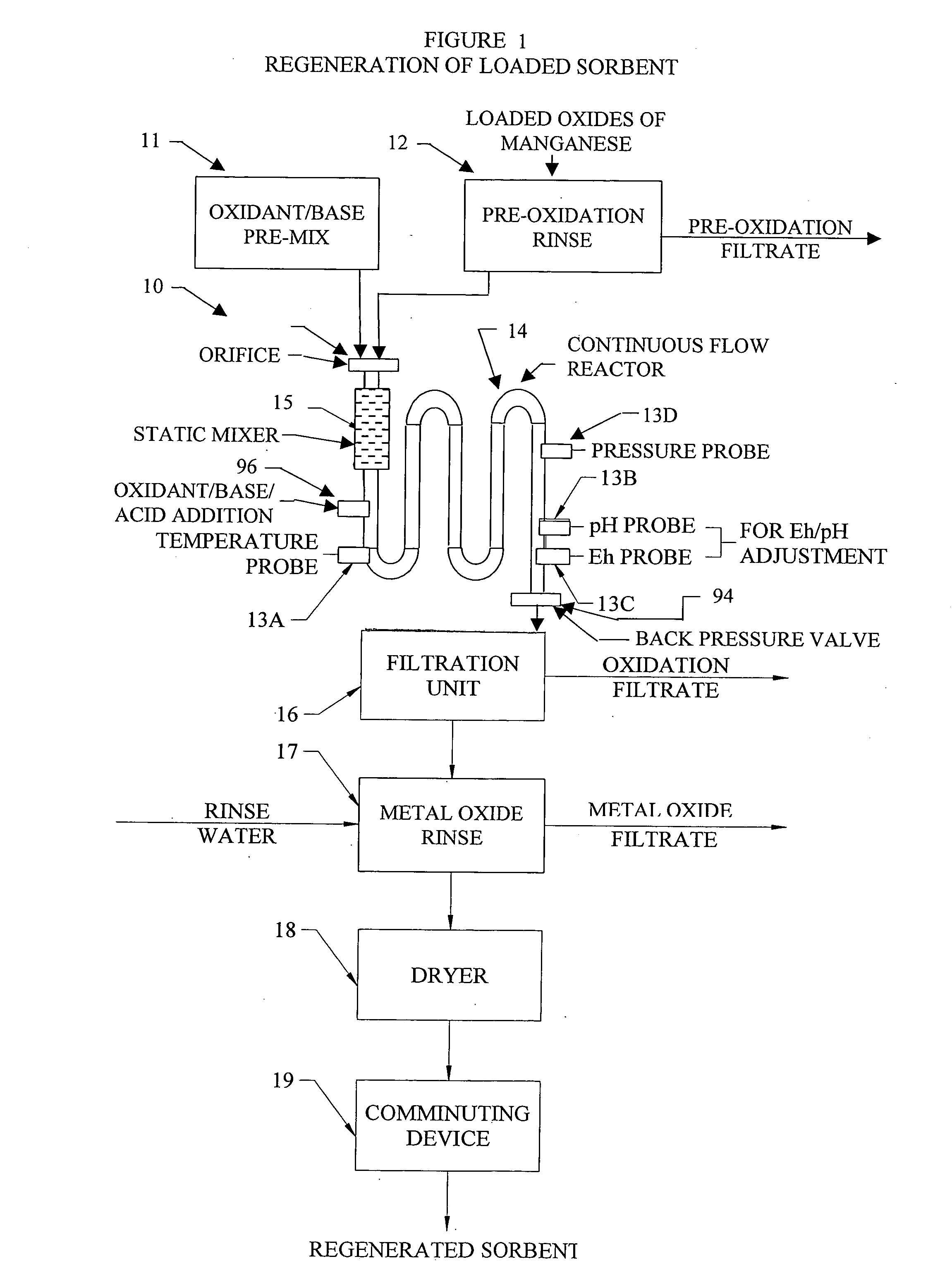
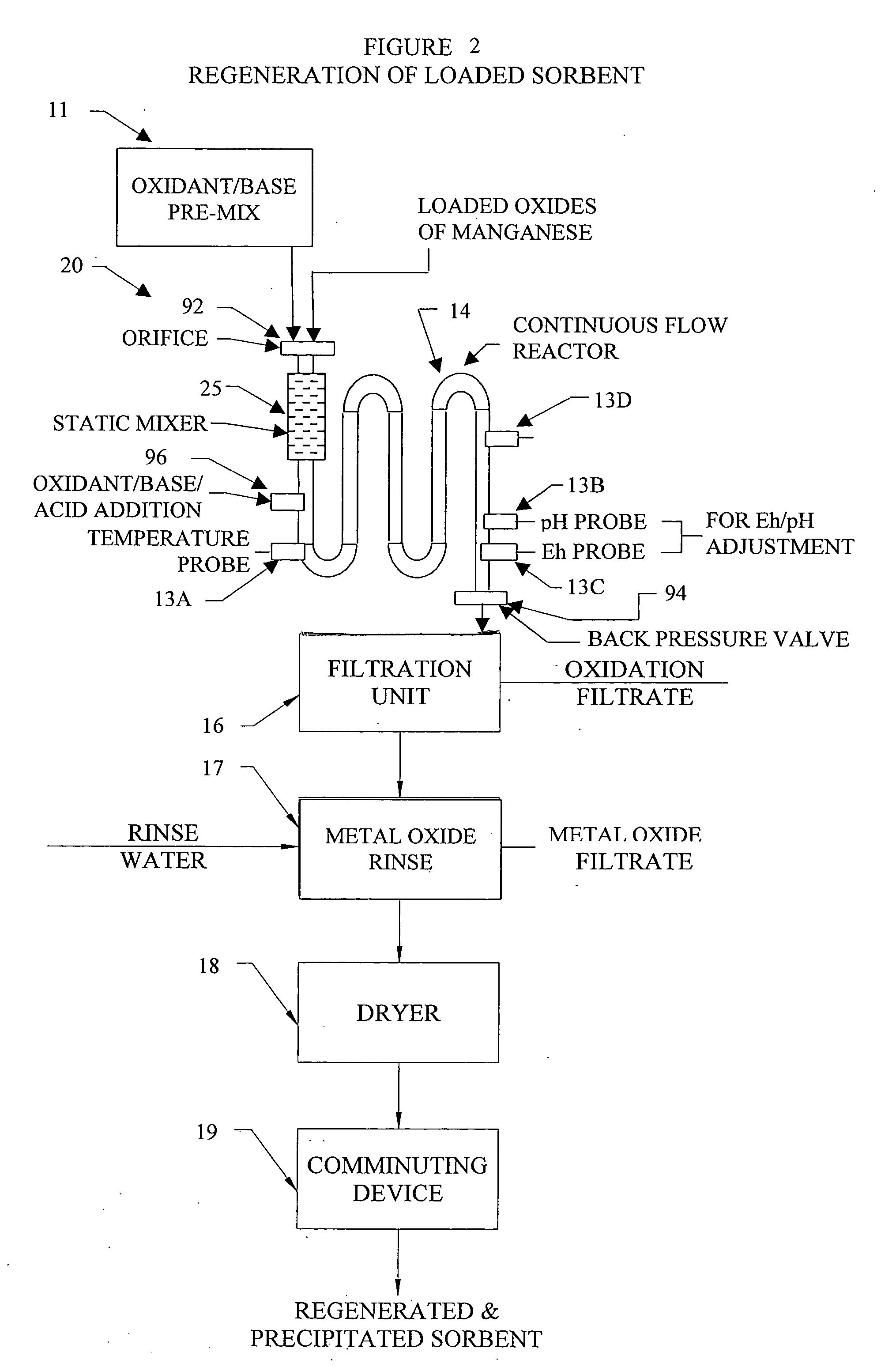
Metal oxide processing methods and systems
InactiveUS20050074380A1Move quicklyIncrease load capacityCombination devicesTemperatue controlIndustrial gasBatch processing
Methods and systems for processing metal oxides from metal containing solutions. Metal containing solutions are mixed with heated aqueous oxidizing solutions and processed in a continuous process reactor or batch processing system. Combinations of temperature, pressure, molarity, Eh value, and pH value of the mixed solution are monitored and adjusted so as to maintain solution conditions within a desired stability area during processing. This results in metal oxides having high or increased pollutant loading capacities and / or oxidation states. These metal oxides may be processed according to the invention to produce co-precipitated oxides of two or more metals, metal oxides incorporating foreign cations, metal oxides precipitated on active and inactive substrates, or combinations of any or all of these forms. Metal oxides thus produced are, amongst other uses; suitable for use as a sorbent for capturing or removing target pollutants from industrial gas streams or drinking water or aqueous streams or for personal protective respirators.
Owner:ENVIROSCRUB TECH CORP
Process of making hydrophobic metal oxide nanoparticles
InactiveUS7081234B1Uniform coatingSulfur compoundsCopper oxides/halidesMetal oxide nanoparticlesTransport layer
A process of treating metal oxide nanoparticles that includes mixing metal oxide nanoparticles, a solvent, and a surface treatment agent that is preferably a silane or siloxane is described. The treated metal oxide nanoparticles are rendered hydrophobic by the surface treatment agent being surface attached thereto, and are preferably dispersed in a hydrophobic aromatic polymer binder of a charge transport layer of a photoreceptor, whereby π—π interactions can be formed between the organic moieties on the surface of the nanoparticles and the aromatic components of the binder polymer to achieve a stable dispersion of the nanoparticles in the polymer that is substantially free of large sized agglomerations.
Owner:XEROX CORP
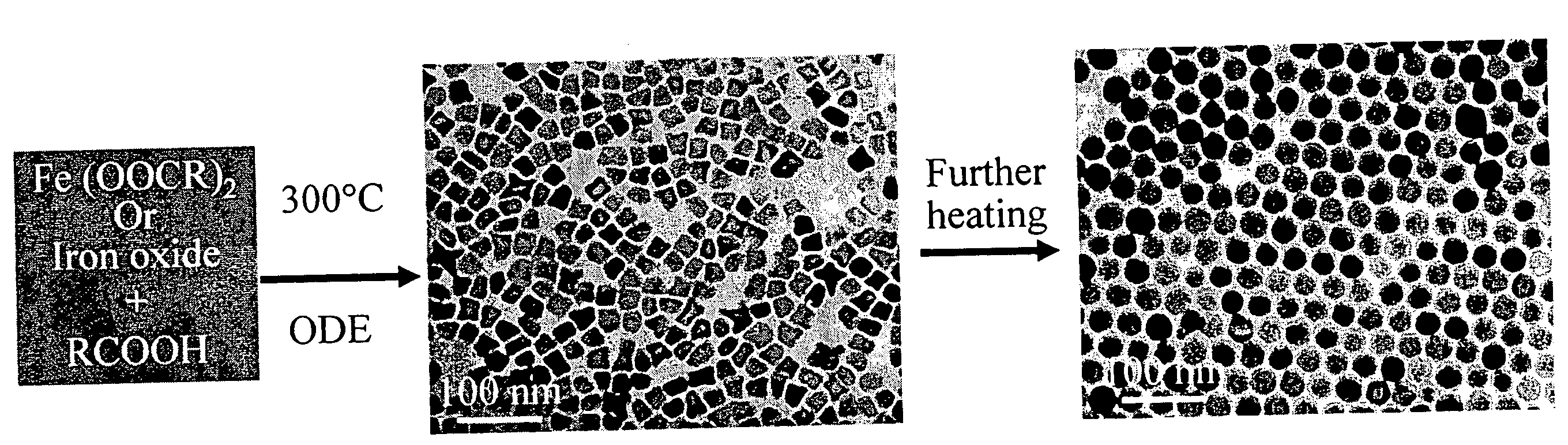
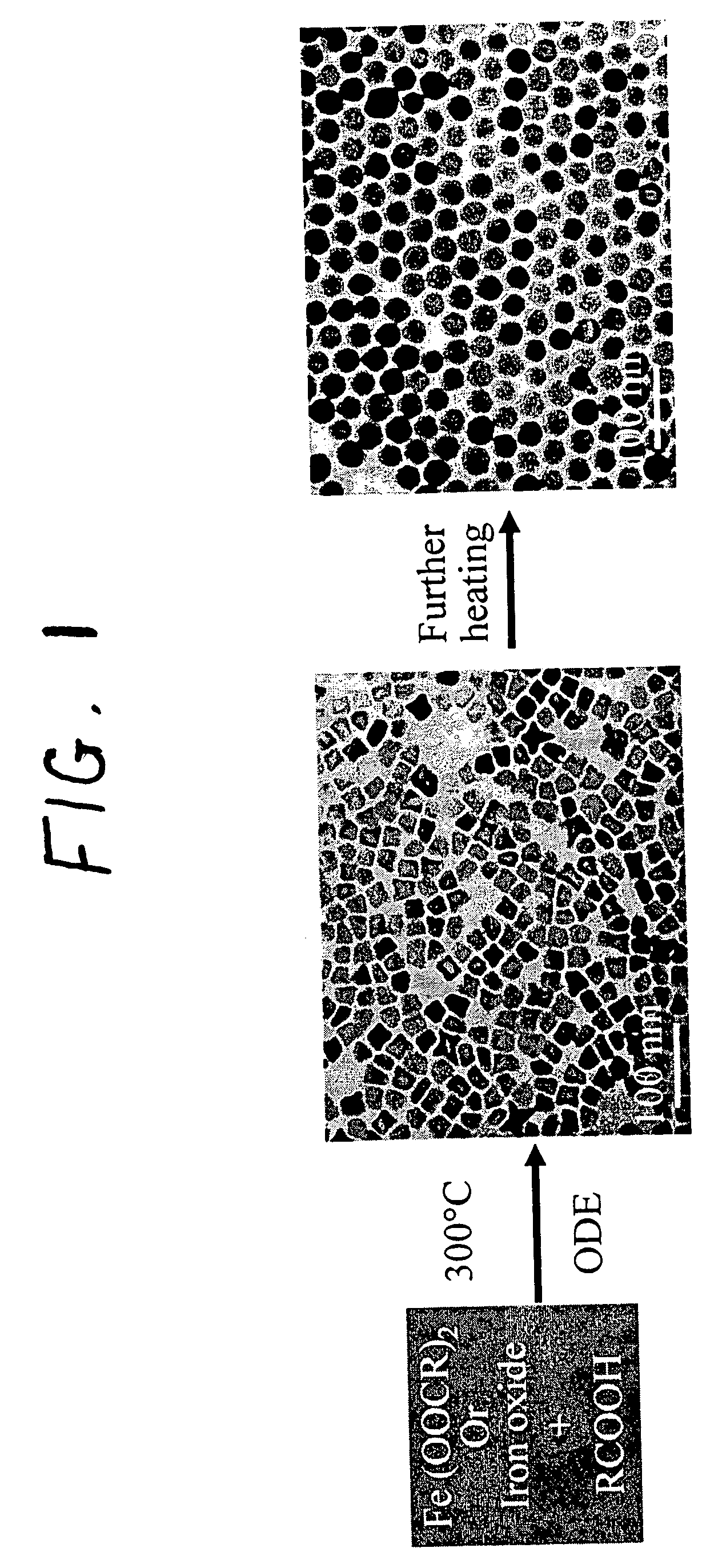
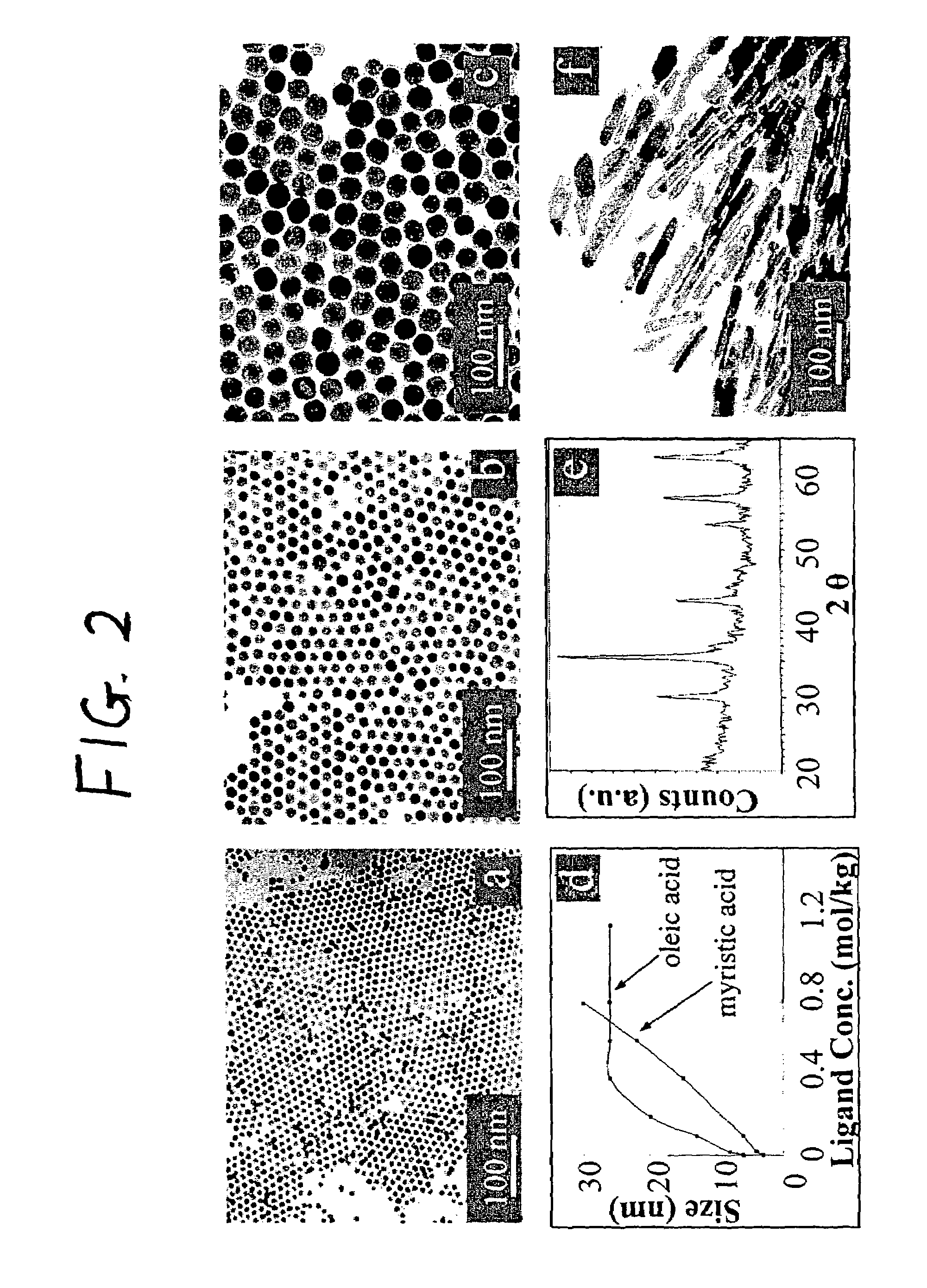
Synthetic control of metal oxide nanocrystal sizes and shapes
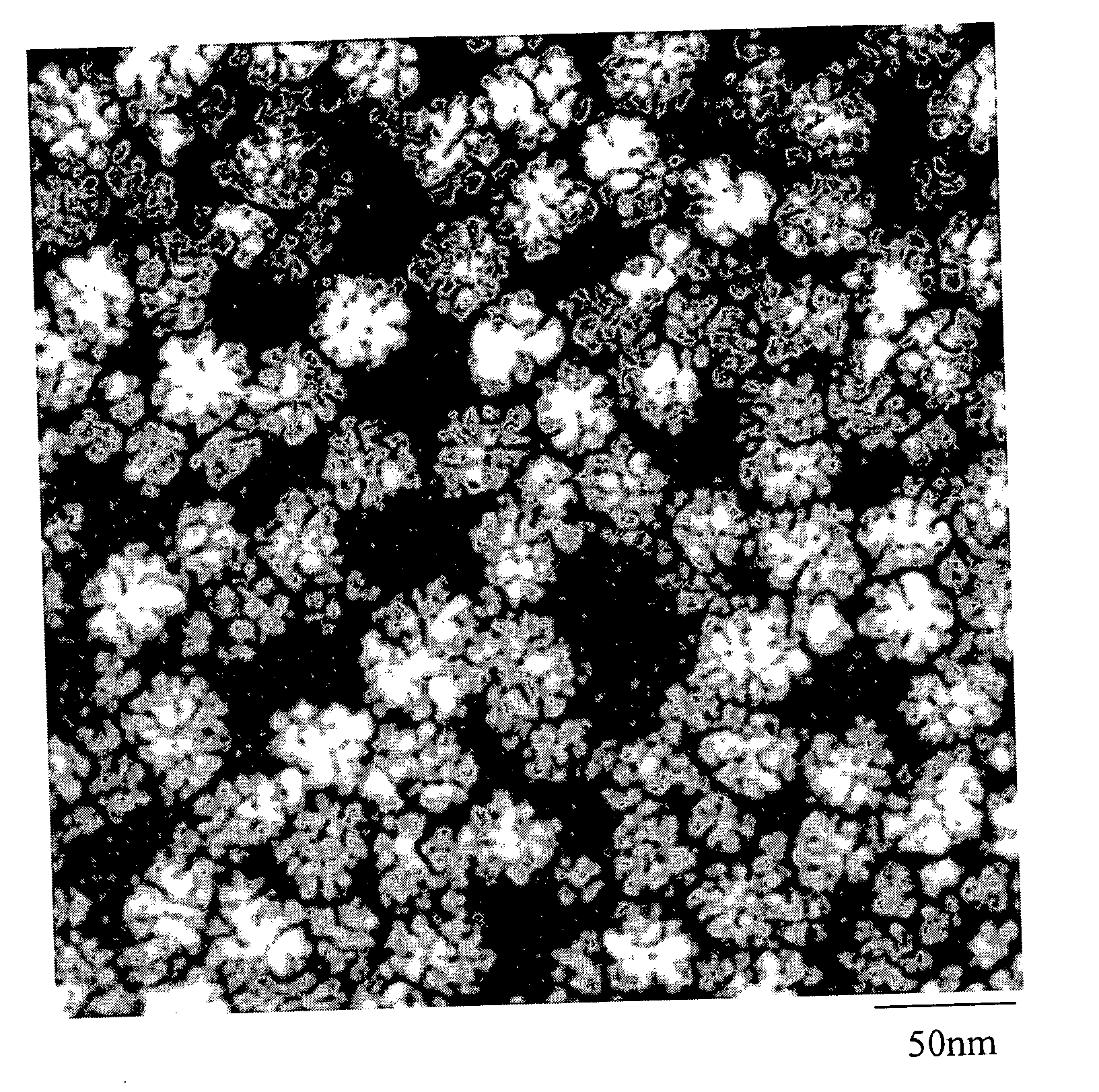
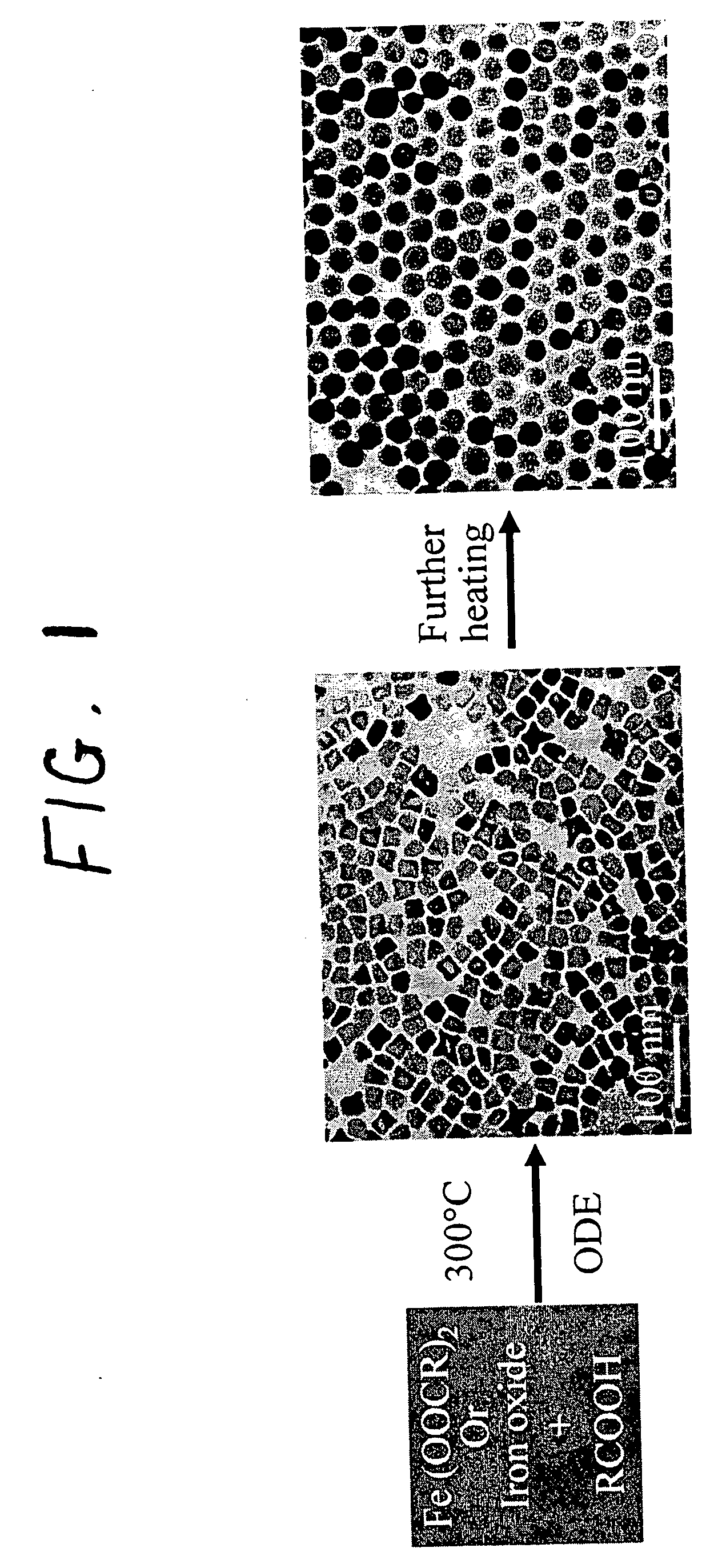
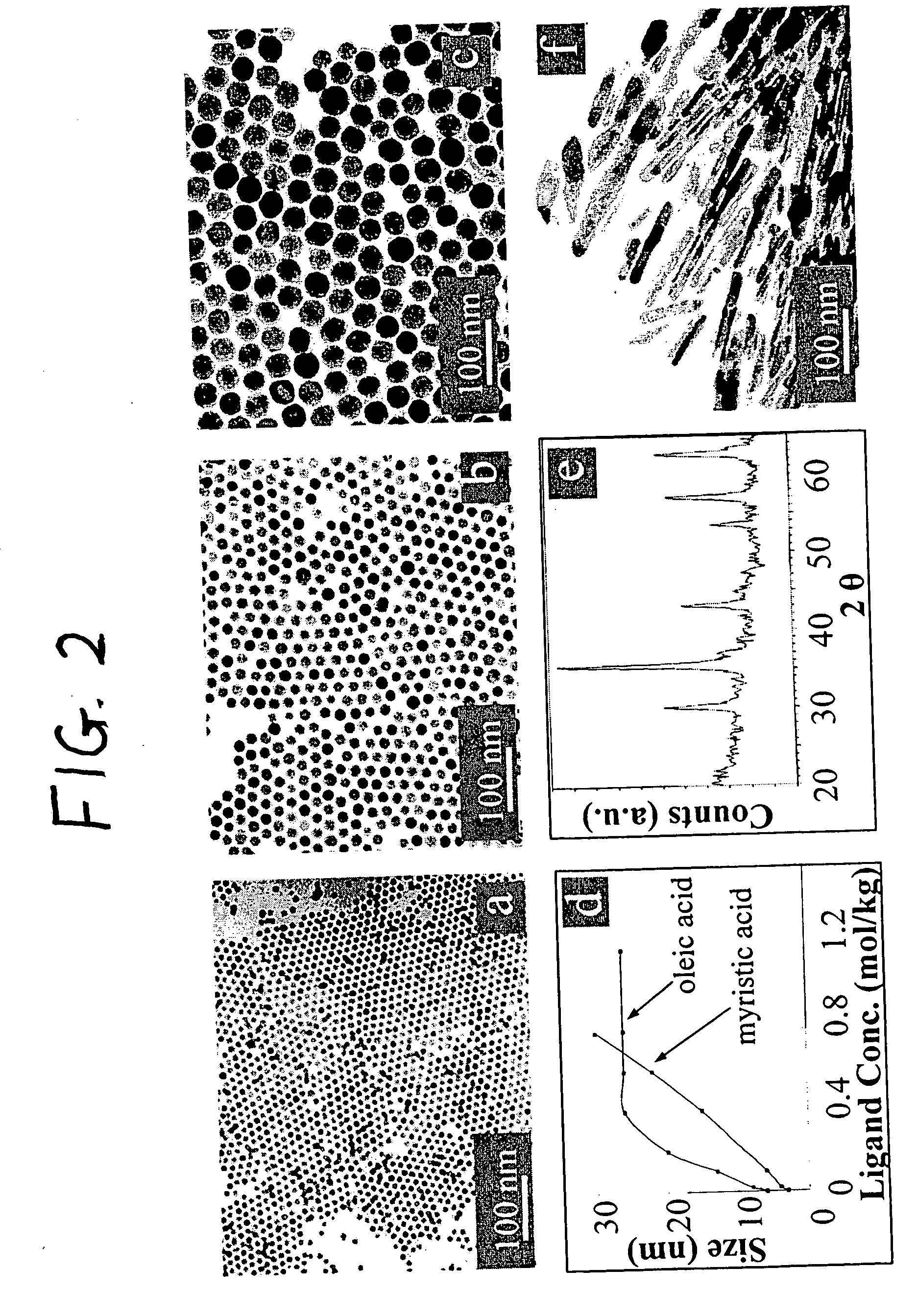
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Methods of Making Binary Metal Oxide Nanostructures and Methods of Controlling Morphology of Same
ActiveUS20100278720A1Reduce crystallinityControl dimensionalityCopper oxides/halidesManganese oxides/hydroxidesPorous membraneNanostructure
The present invention includes a method of producing a crystalline metal oxide nanostructure. The method comprises providing a metal salt solution and providing a basic solution; placing a porous membrane between the metal salt solution and the basic solution, wherein metal cations of the metal salt solution and hydroxide ions of the basic solution react, thereby producing a crystalline metal oxide nanostructure.
Owner:WONG STANISLAUS S +1
Processing method for stainless steel acid cleaning waste water and liquid
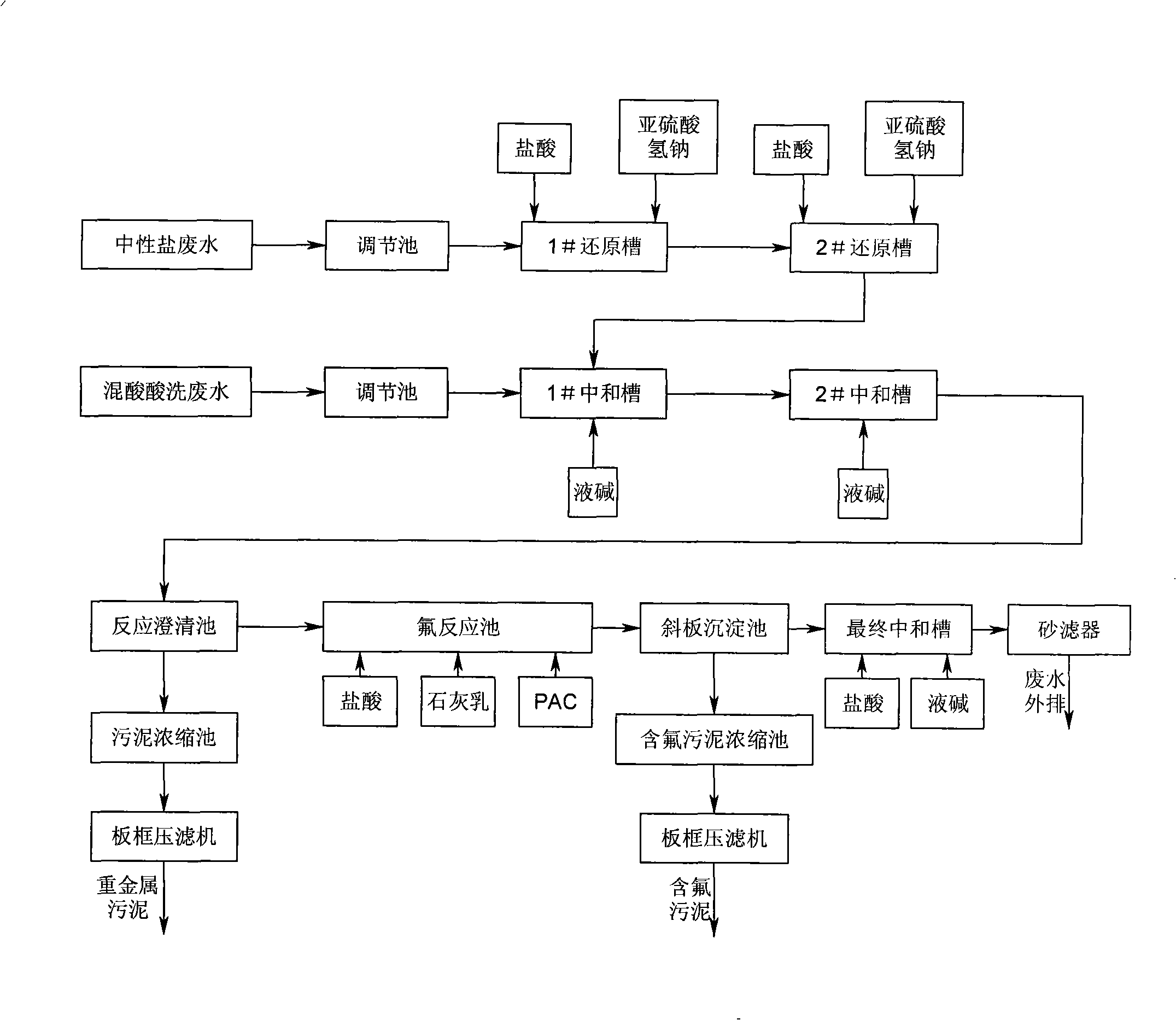
InactiveCN101269889AMeet emission compliance requirementsMeet compliance requirementsSludge treatment by de-watering/drying/thickeningIron oxides/hydroxidesLiquid wasteSludge
The invention relates to a processing method of stainless steel pickling waste liquid, which is characterized in that the processing method includes the steps that: neutral salt wastewater flows into a neutral reduction cell, and mixed acid pickling waste waters flow into a neutralizing tank; reducing agent is added into the neutral reduction cell, and then the reduced wastewater liquid is put into the neutralizing tank and is mixed with the adjusted mixed acid pickling waste waters, and liquid alkali is added into the neutralizing tank; the separated clear waste liquid removes fluorin ions in a settlement tank; neutralizing liquid is added into a final neutralizing tank to cause the pH value of treatment liquid to adjust to be neutralized wastewater liquid which passes through a sand filter to be discharged. The processing method has the advantages that metal ions and fluorin ions are performed the subsection treatment, and neutralizing agent is changed into the liquid alkali from lime cream, the requirement of reaching the standard of the waste liquid emission is not only achieved, but also the mud quantity produced by a mass of lime cream is greatly reduced, heavy metal mud of a retention pond only contains heavy metal compound, the salts of calcium fluoride and calcium sulphate, etc. are separated from the inclined plate settlement tank during the fluoridation stage, thereby the treatment cost is effectively lowered.
Owner:NINGBO BAOXIN STAINLESS STEEL
Oxidative Treatment Method
InactiveUS20100320156A1Quickly and efficiently decomposeEffective treatmentWater/sewage treatment by irradiationDecorative surface effectsElectrolysisWaste stream
The present invention provides a method for oxidizing a substance (e.g., in a waste stream, drinking water, a paper pulp slurry, or on a surface), which uses free radicals and reactive species generated from multiple oxidants. The method comprises combining peroxynitrite or peroxynitrous acid and at least one additional oxidizing agent for a period of time sufficient to oxidize the substance of interest. The peroxynitrite or peroxynitrous acid preferably is formed by irradiation of nitrate ion and / or nitric acid (e.g., with UV or gamma rays). The yield of free radicals and reactive species, which are the intermediate species that perform the oxidation may be increased by addition of a catalysts, electromagnetic radiation, sonic waves, and / or electrolysis.
Owner:TULANE EDUCATIONAL FUND
Synthetic control of metal oxide nanocrystal sizes and shapes
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Method of producing inorganic aerogels under subcritical conditions
PCT No. PCT / EP96 / 04250 Sec. 371 Date Apr. 14, 1998 Sec. 102(e) Date Apr. 14, 1998 PCT Filed Sep. 30, 1996 PCT Pub. No. WO97 / 13721 PCT Pub. Date Apr. 17, 1997Preparation of inorganic aerogels based on oxides of the metals magnesium, aluminum, silicon, tin, lanthanum, titanium, zirconium, chromium and / or thorium by producing a hydrogel in a sol / gel process, replacing the water in the hydrogel by an organic solvent, and drying the solvent-moist gel, takes place by conducting the drying step by exposing the solvent-moist gel to an ambient temperature which is above the boiling temperature of the solvent and at a pressure which is below the supercritical pressure of the solvent in such a way that the solvent within the gel is heated up very rapidly and evaporates.
Owner:BASF AG
Multiferroic element
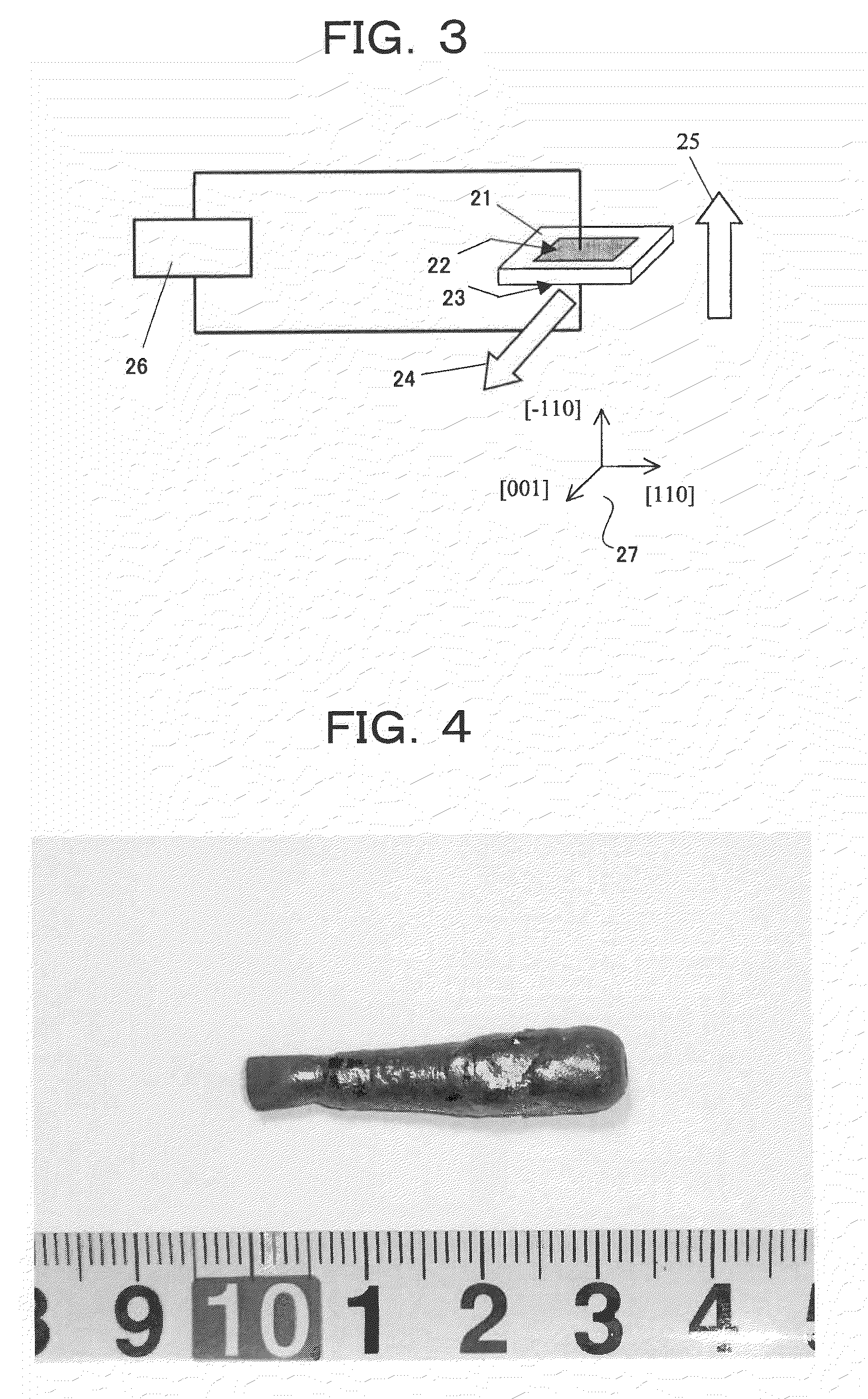
InactiveUS20090196818A1Simple structureSolid-state devicesGalvano-magnetic material selectionMagnetizationMultiferroics
A multiferroic element having a simple structure in which orientation of electric polarization or magnetization of a solid state material can be controlled by applying a magnetic field or an electric field, respectively. By applying an external magnetic field to a multiferroic solid state material that exhibits ferroelectricity and ferromagnetism having a spin structure such that the orientation of spin is rotating along the outside surface of a cone (apex angle alpha at the top of the cone is in a range of 0<alpha<=90 degrees), an electric polarization with orientation substantially perpendicular to the direction of the externally applied magnetic field can be controlled. Meanwhile, by applying an external electric field to the multiferroic solid state material, a magnetization with an orientation substantially perpendicular to the direction of the externally applied electric field can be controlled.
Owner:JAPAN SCI & TECH CORP +1
Process for simultaneous recovery of chromium and iron from Chromite Ore Processing Residue
InactiveUS20040086438A1Simple eco-friendlySolvent extractionMolybdeum compoundsIron saltsChromate salt
The present invention relates to a process for simultaneous recovery of chromium and iron from Chromite Ore Processing Residue (COPR) and more particularly, the present invention relates to an economical and environment-friendly process for recovering chromium as a chromate salt and iron as an iron salt from non-leachable Chromite Ore Processing Residue and avoids landfilling of toxic metals.
Owner:COUNCIL OF SCI & IND RES
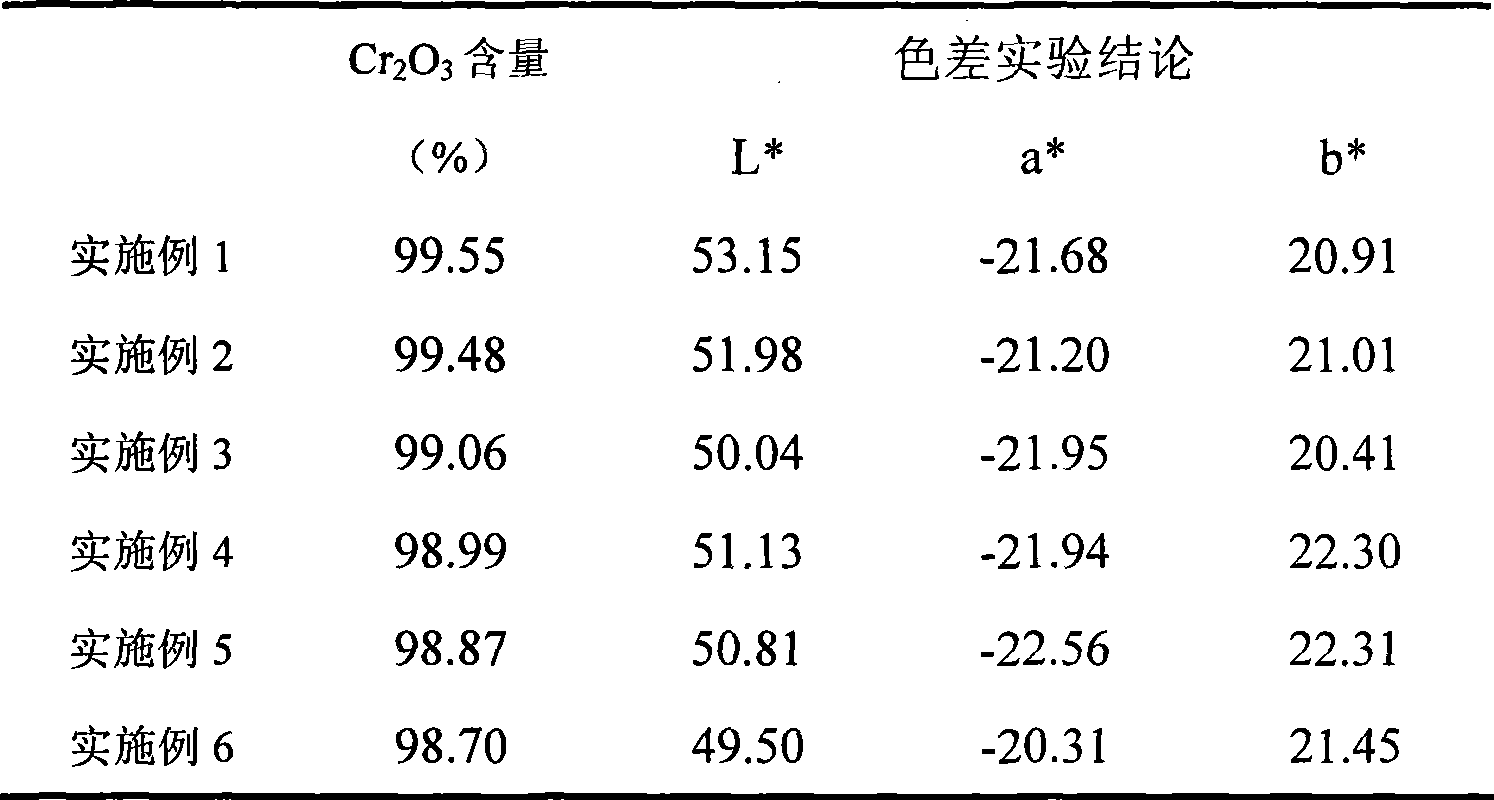
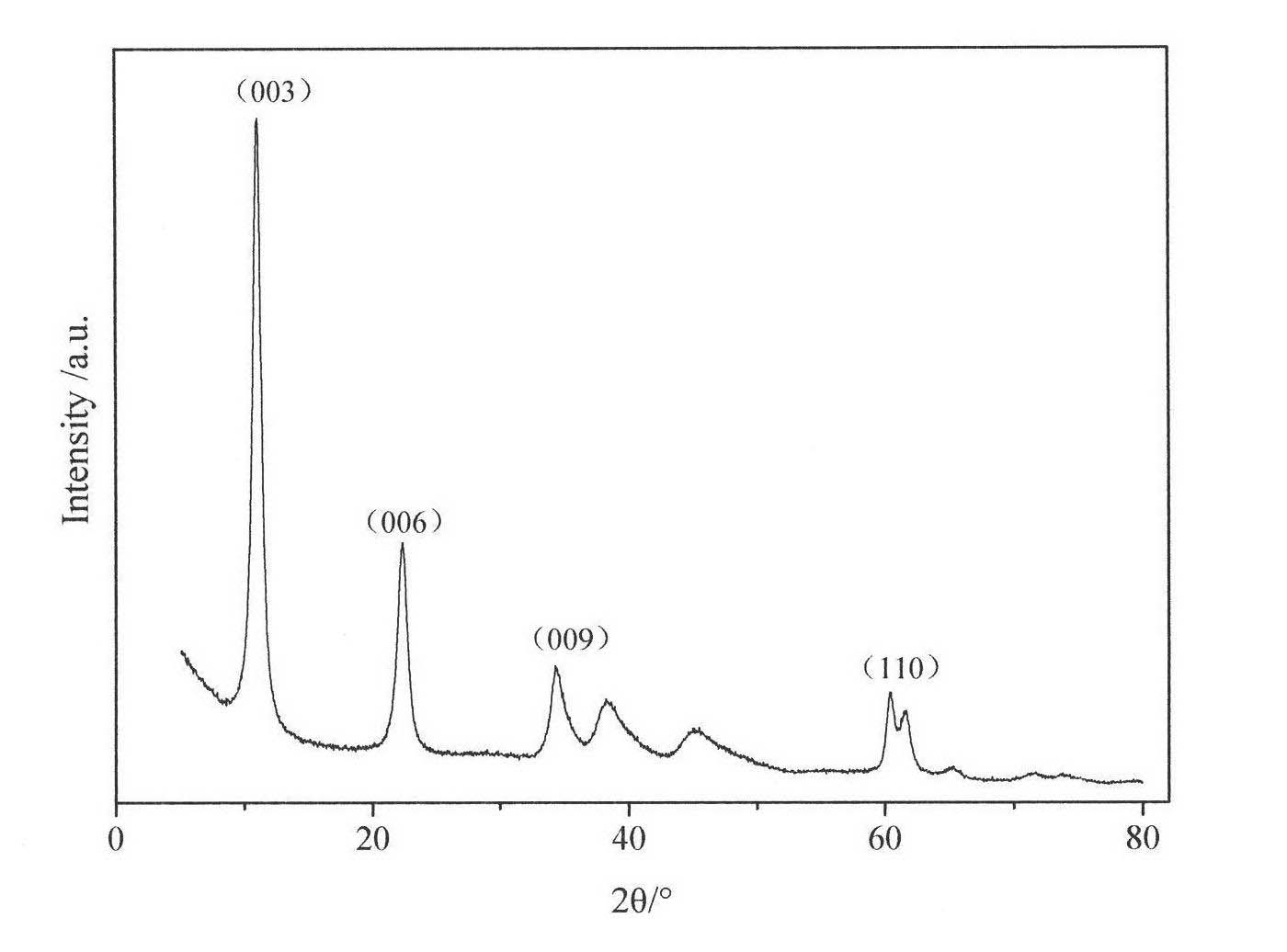
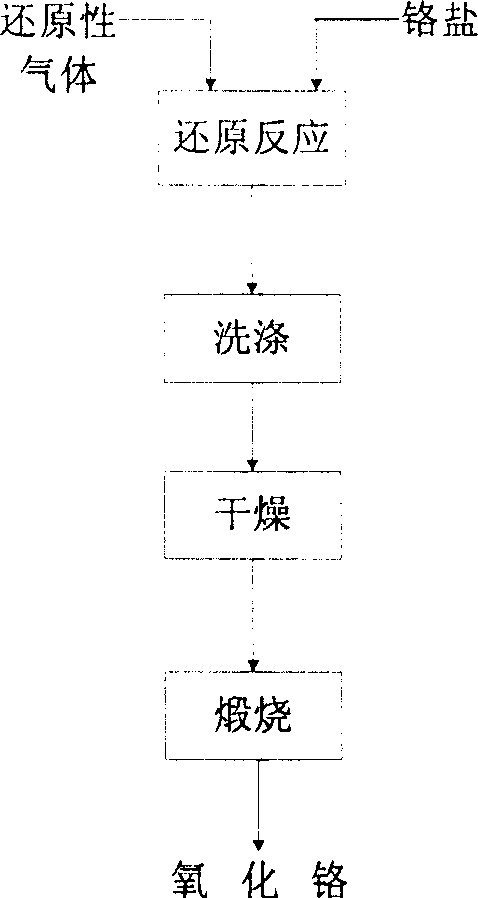
Method for preparing pigment grade chromium hemitrioxide green by using gaseous reducing agent low temperature reduction chromate salt
The invention discloses a method for preparing pigment-grade chromium oxide green. The method comprises the steps of taking reducing gas as a reducing agent, allowing chromic salt to react with the reducing gas for 0.5 to 3 hours at a temperature between 300 and 800 DEG C, cooling, using water to wash and hydrolyzing a reaction mixture, mixing the reaction mixture with additives at a temperature between 700 and 1,300 DEG C, activating and sintering the reaction mixture and the additives for 0.5 to 3 hours, washing, drying and grading the sintered material to obtain the pigment-grade chromium oxide green, wherein the chromic salt is chromate or bichromate; the reducing gas is hydrogen, natural gas, ammonia gas, coal gas or a mixture thereof; and the additives are silicon compounds, titanium compounds, boron compounds, phosphorus compounds, aluminum compounds, barium compounds, zinc compounds and other compounds, such as SiO2, K2SiO3, Na2SiO3, TiO2, K2TiO3, B2O3, H2BO3, H3PO4, P2O3, Al2O3, Al(OH)3, Al2(SO4)3, AlCl3, Al(NO3)3, BaO, Ba(OH)2, BaSO4, BaCl2, Ba(NO3)2, ZnO, ZnCl2, ZnSO4, Zn(NO)3, Sb2O3 and other substances.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for preparing hydrotalcite
InactiveCN101817510AGuaranteed grain size uniformityReduce pH fluctuationsOxide/hydroxide preparationIron oxides/hydroxidesHydrotalciteBuffer solution
The invention relates to a method for preparing hydrotalcite, which comprises the following steps of: (1) preparation of buffer solution, namely preparing the buffer solution with a pH value of 9 to 10 by using aqueous ammonia and ammonium chloride or aqueous ammonia and ammonium bicarbonate; (2) dripping of metal salt solution, namely dripping the metal salt solution into the stirred buffer solution, and continuously stirring the solution for 1 to 1.5 hours after the dripping is finished; (3) crystallization and washing, namely crystallizing the stirred mixed solution for 6 to 24 hours at the temperature of between 55 and 95 DEG C, and filtering and washing the crystallized solution to obtain filter solution and filter cakes; and (4) drying and grinding, namely drying the filter cakes at the temperature of between 55 and 95 DEG C and grinding the filter cakes into powder. The method provides a stable solution environment with a pH value of 9 to 10 for the formation of the hydrotalcite by using the buffer solution used as a precipitating agent, ensures complete crystal phase structure and uniform grain size of a hydrotalcite product, and solves the problems of difficult pH value control, wide grain size distribution and the like during preparing the hydrotalcite by using a traditional co-precipitation method in the industry.
Owner:HUZHOU TEACHERS COLLEGE
Method of preparing chromium oxide by reducing chromate with gaseous reducing agent at low temperature
InactiveCN1907865ASimple production processEasy to industrializeChromium oxides/hydratesHydrogenReaction temperature
This invention involves a preparation method for chromium oxide with chromium salt as raw materials and reducing gas as a reductant. Said method comprises carrying out reaction of chromium salt with superfluous reducing gas at 300~850DEG C for 0.5-3 h and cooling to obtain a reaction mixture, washing the mixture and drying, and calcining at 400~1100DEG C for 1-3 h to obtain the ultrafine chromium oxide powders, wherein the chromium salt is chromate or dichromate, and the reducing gas is hydrogen, natural gas, gas or their mixtures. Compared with existing technologies, this invention has the advantages of simple production process and being easy for industrialization. The obtained chromium oxide has high purity, uniform particle size distribution and small particle size. The whole process does not produce containing chromium-containing waste, and is environmental friendly. The reaction by-product can be reused, which greatly reduces the cost of production. Low reaction temperature greatly reduces the reaction energy.
Owner:中蓝义马铬化学有限公司
Ultraviolet light screening compositions
InactiveUS6869596B1Minimize migrationReduce productionMaterial nanotechnologyCosmetic preparationsUltraviolet lightsUltraviolet A Radiation
Owner:OXFORD UNIV INNOVATION LTD
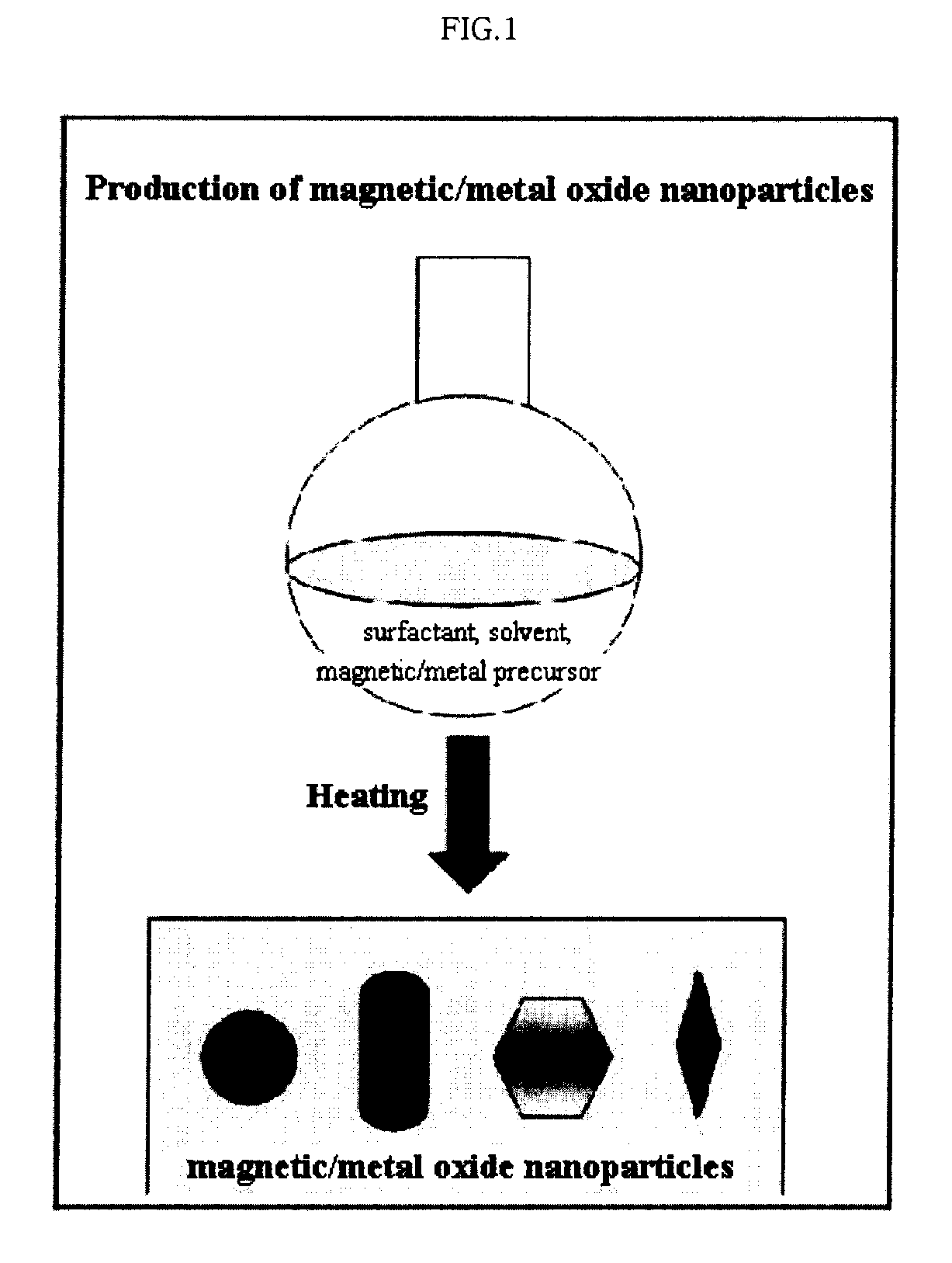
Preparation Method of Magnetic and Metal Oxide Nanoparticles
ActiveUS20080003159A1Efficient mass productionUniform shapeCopper oxides/halidesManganese oxides/hydroxidesMetal oxide nanoparticlesMagnetic oxide
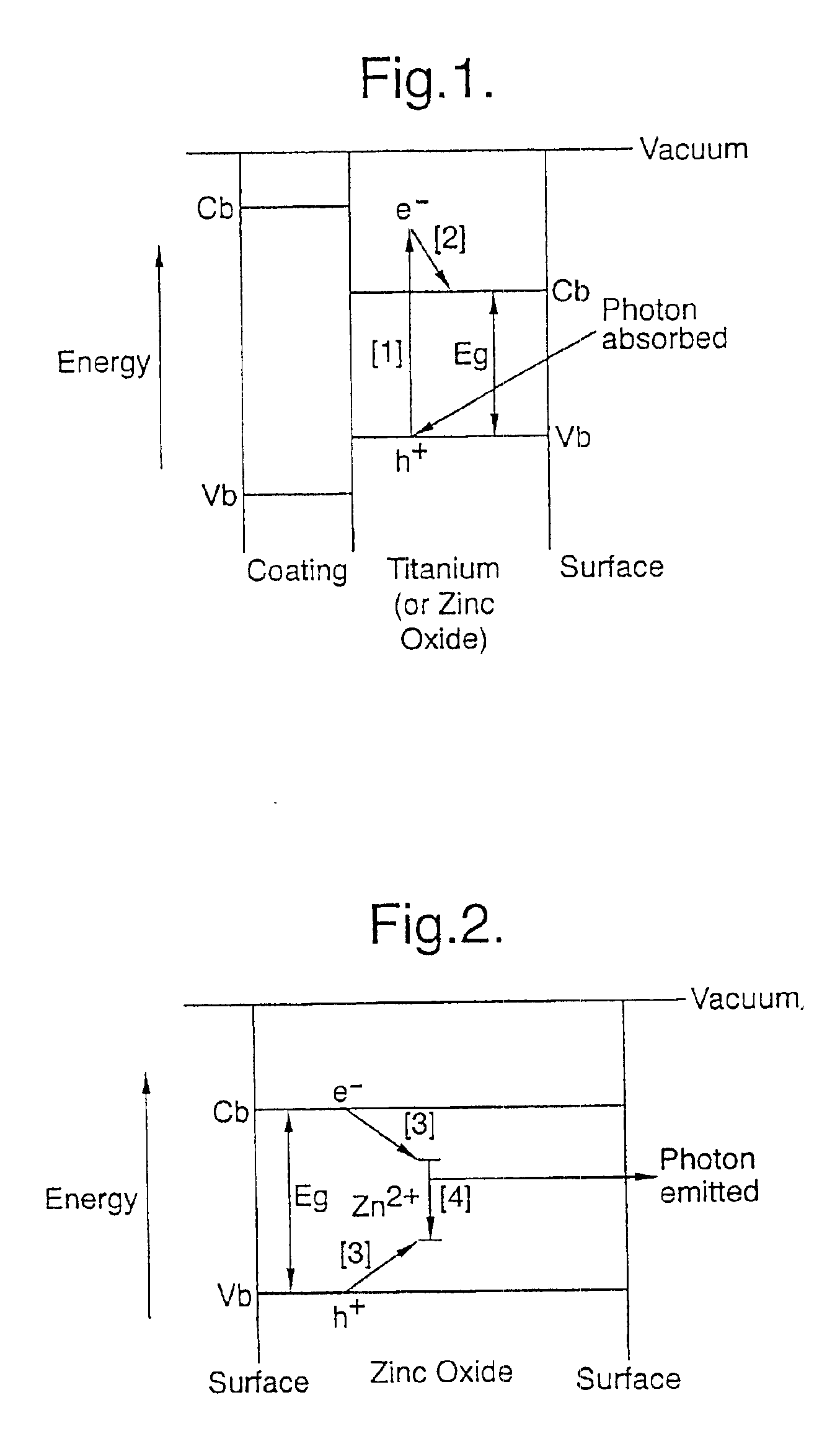
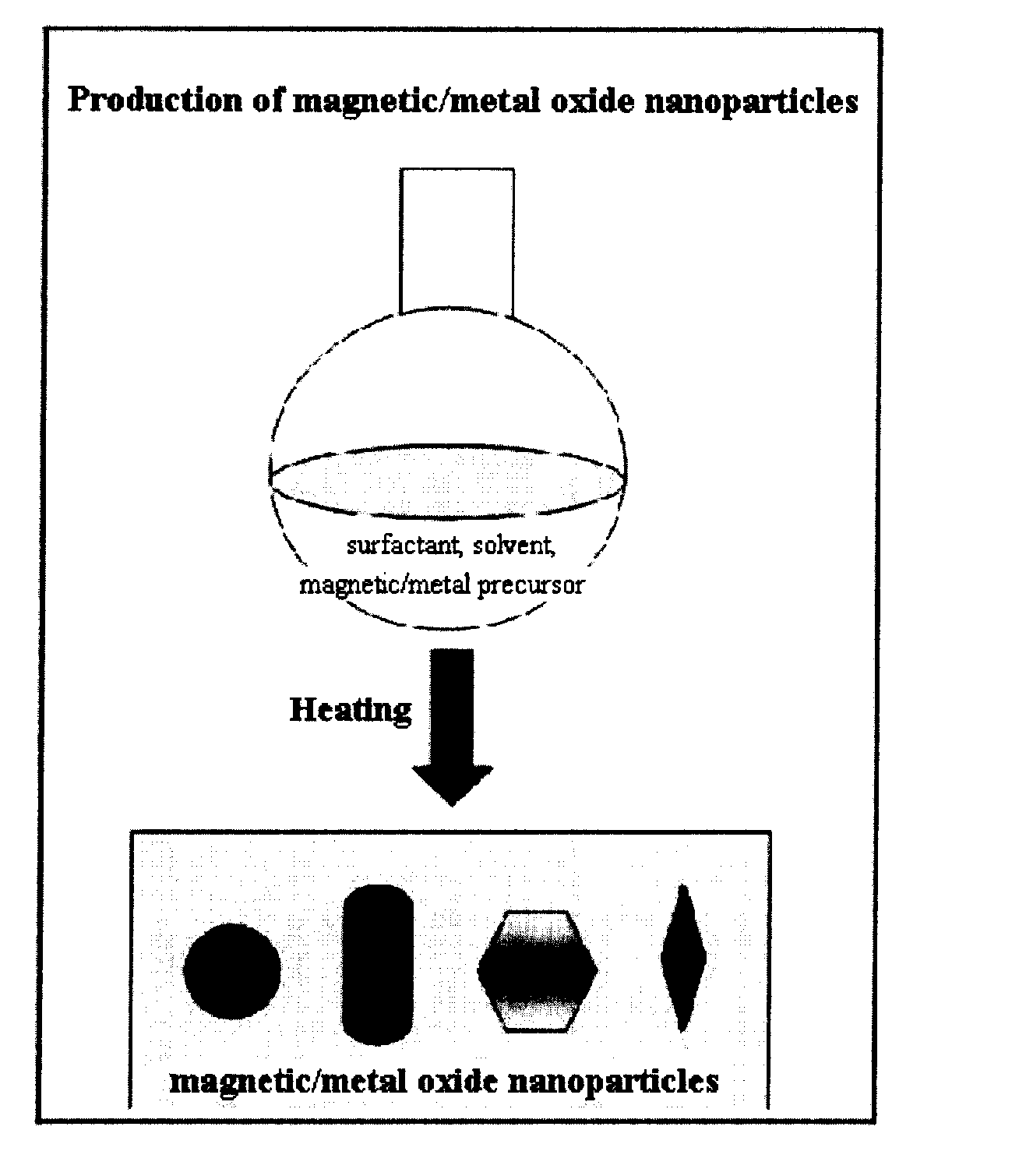
This invention relates, in general, to a method of producing magnetic oxide nanoparticles or metal oxide nanoparticles and, more particularly, to a method of producing magnetic or metal oxide nanoparticles, which comprises (1) adding a magnetic or metal precursor to a surfactant or a solvent containing the surfactant to produce a mixed solution, (2) heating the mixed solution to 50-6001 C to decompose the magnetic or metal precursor by heating so as to form the magnetic or metal oxide nanoparticles, and (3) separating the magnetic or metal oxide nanoparticles. Since the method is achieved through a simple process without using an oxidizing agent or a reducing agent, it is possible to simply mass-produce uniform magnetic or metal oxide nanoparticles having desired sizes compared to the conventional method.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Process for preparing chromic oxide by hydrothermal reducing sodium chromate or sodium acid chromate
InactiveCN101456588AHigh reduction conversion rateAchieve recyclingChromium oxides/hydratesSlurryHigh pressure
The invention relates to technology for preparing chromium oxide through hydrothermal reduction of sodium chromate or sodium dichromate, which comprises: preparing the sodium chromate or the sodium dichromate into an aqueous solution, placing the aqueous solution and starch or starch derivatives into a high-pressure reaction kettle for hydrothermal reduction, and obtaining chromic hydroxide slurry; filtering and separating the chromic hydroxide slurry to obtain chromic hydroxide filter cakes and a chromic hydroxide mother solution; and performing high-temperature roasting on the filter cakes to obtain chromium oxide products. The technology has short technological flow, simple operation, low cost and mild reaction conditions, and does not have overhigh requirements on equipment; not only the product quality is better than that of the prior method but also the reduction and conversion rate of sexavalent chromium reaches more than 98 percent; and the whole technology forms a closed cycle, realizes recycling of mixed alkali, the mother solution and a washing liquid, does not discharge pollutant in any form during the whole process, and fundamentally avoids pollution.
Owner:CENT SOUTH UNIV
Metal oxide powder and method for the production of the same
InactiveUS6303091B1Industrially advantageousTantalum compoundsFerric oxidesGranularityParticle-size distribution
A metal oxide powder except alpha-alumina, is disclosed comprising polyhedral particles having at least 6 planes each, a number average particle size of from 0.1 to 300 mum, and a D90 / D10 ratio of 10 or less where D10 and D90 are particle sizes at 10% and 90% accumulation, respectively from the smallest particle size side in a cumulative particle size curve of the particles. This metal oxide powder contains less agglomerated particles, and has a narrow particle size distribution and a uniform particle shape.
Owner:SUMITOMO CHEM CO LTD
Process for producing metal oxide flakes
InactiveUS20070243337A1Material nanotechnologyPigment preparation by wet methodsDielectric layerMaterials science
The present invention relates to a process for the preparation of a plane-parallel structure (a platelet-shaped body, or flake), comprising at least one dielectric layer consisting of oxides of one or more metal selected from groups 3 to 15 of the periodic table, which method comprises: (a) optionally applying a layer of release material on a substrate, (b) applying a composition comprising one or more precursors of one or more desired metal oxides to said release layer, or directly to a substrate with no layer of release material, (c) subjecting the one or more precursors of one or more desired metal oxides to microwave radiation to form a metal oxide layer on the substrate or on the layer of release material; and (d) separating the resulting metal oxide layer from the substrate as plane-parallel structures.
Owner:BASF AG
Method of producing nano-scaled graphene and inorganic platelets and their nanocomposites
ActiveUS7892514B2Readily captured and re-usedReduce impactCarbon compoundsSelenium/tellurium compundsLiquid mediumHalogen
Disclosed is a method of exfoliating a layered material (e.g., graphite and graphite oxide) to produce nano-scaled platelets having a thickness smaller than 100 nm, typically smaller than 10 nm, and often between 0.34 nm and 1.02 nm. The method comprises: (a) subjecting the layered material in a powder form to a halogen vapor at a first temperature above the melting point or sublimation point of the halogen at a sufficient vapor pressure and for a duration of time sufficient to cause the halogen molecules to penetrate an interlayer space of the layered material, forming a stable halogen-intercalated compound; and (b) heating the halogen-intercalated compound at a second temperature above the boiling point of the halogen, allowing halogen atoms or molecules residing in the interlayer space to exfoliate the layered material to produce the platelets. Alternatively, rather than heating, step (a) is followed by a step of dispersing the halogen-intercalated compound in a liquid medium which is subjected to ultrasonication for exfoliating the halogen-intercalated compound to produce the platelets, which are dispersed in the liquid medium. The halogen can be readily captured and re-used, thereby significantly reducing the impact of halogen to the environment. The method can further include a step of dispersing the platelets in a polymer or monomer solution or suspension as a precursor step to nanocomposite fabrication.
Owner:GLOBAL GRAPHENE GRP INC
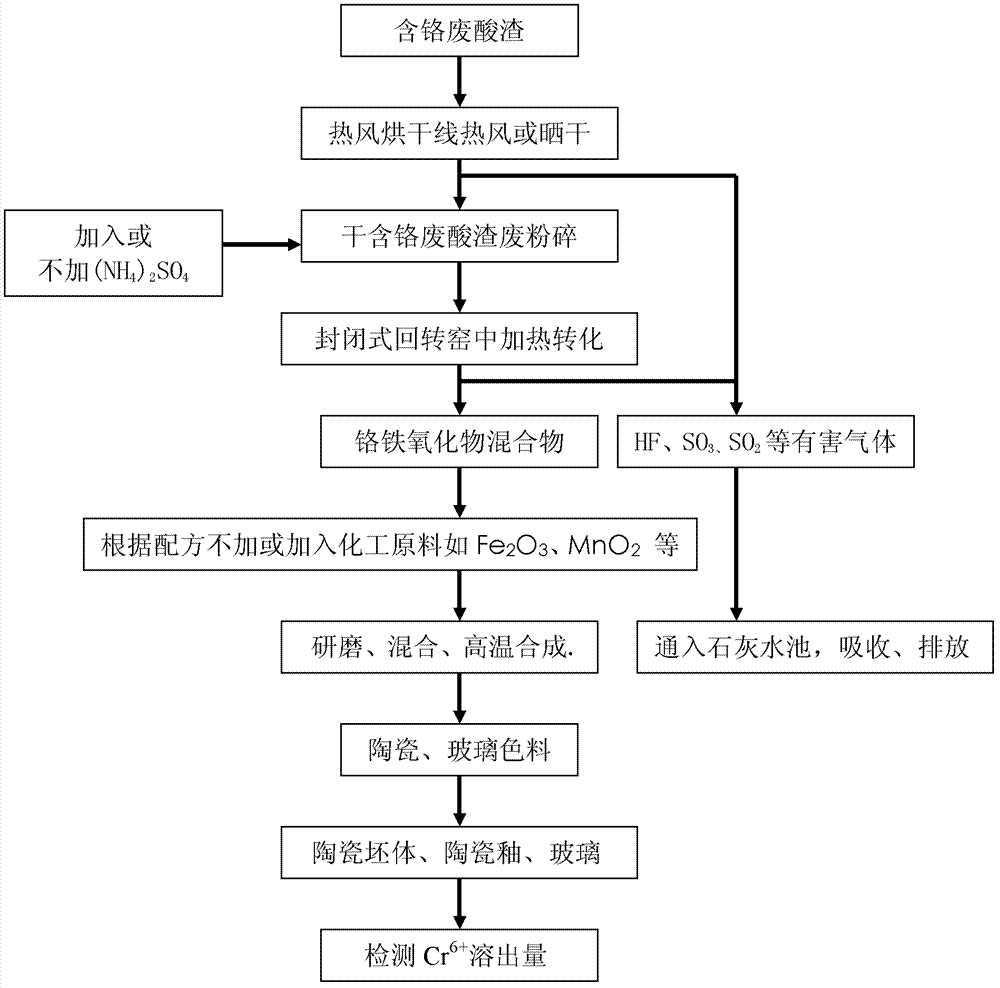
Application treatment method of chromium-containing waste acid sludge in stainless steel factory
An application treatment method of chromium-containing waste acid sludge in stainless steel factories particularly comprises the following steps: 1) drying chromium-containing waste acid sludge generated during stainless steel production, passivation, and pickling technology processes at 80-20o DEG C by hot air or in the sun; 2) directly putting the dried chromium-containing waste acid sludge in a closed rotary kiln for heating reaction, or adding (NH4)2SO4 and then putting into the closed rotary kiln for heating reaction so as to allow all the acid sludge to convert into a ferrochromium oxide mixture, introducing the tail gas into limewash for tail gas harmless treatment; 3) directly using the ferrochromium oxide mixture as a ceramic pigment or a glass pigment, or using the ferrochromium oxide mixture as a raw material for synthesizing a ceramic pigment or a glass pigment. The invention eliminates the harm of chromium, saves resources, reduces the production cost of ceramic glass pigments, also reduces environment pollution, and changes waste into valuables.
Owner:佛山市中天荣环保新材料科技有限公司 +2
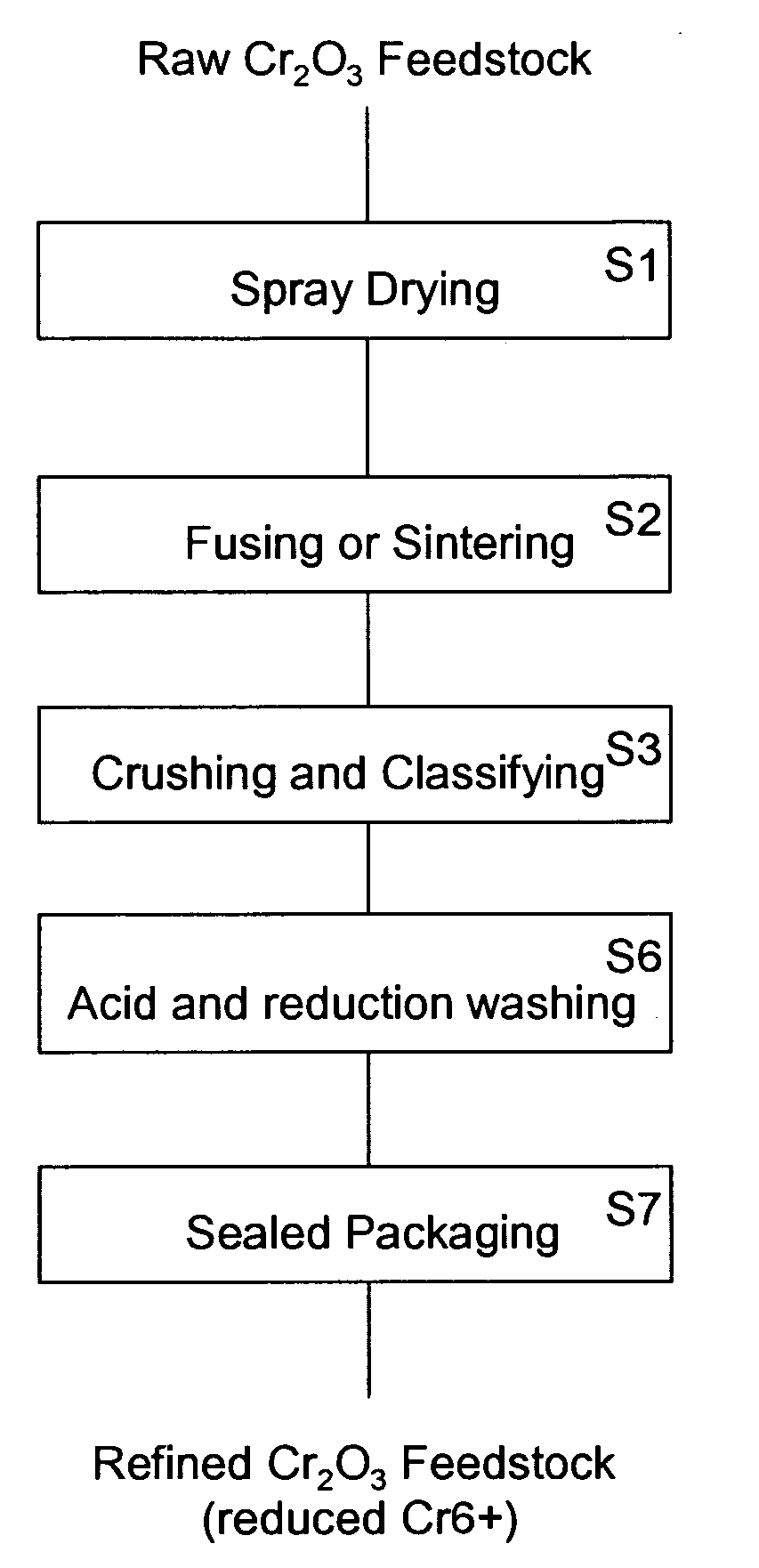
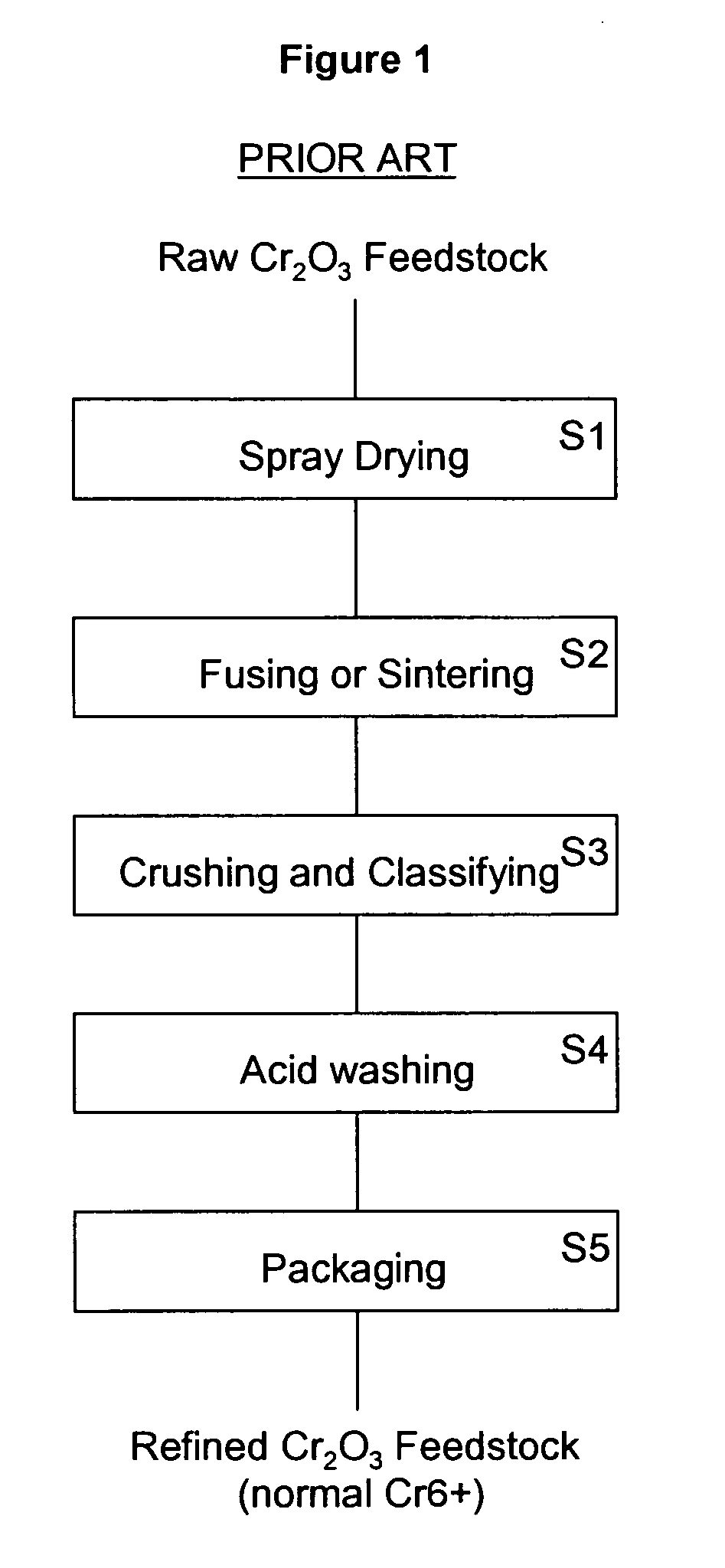
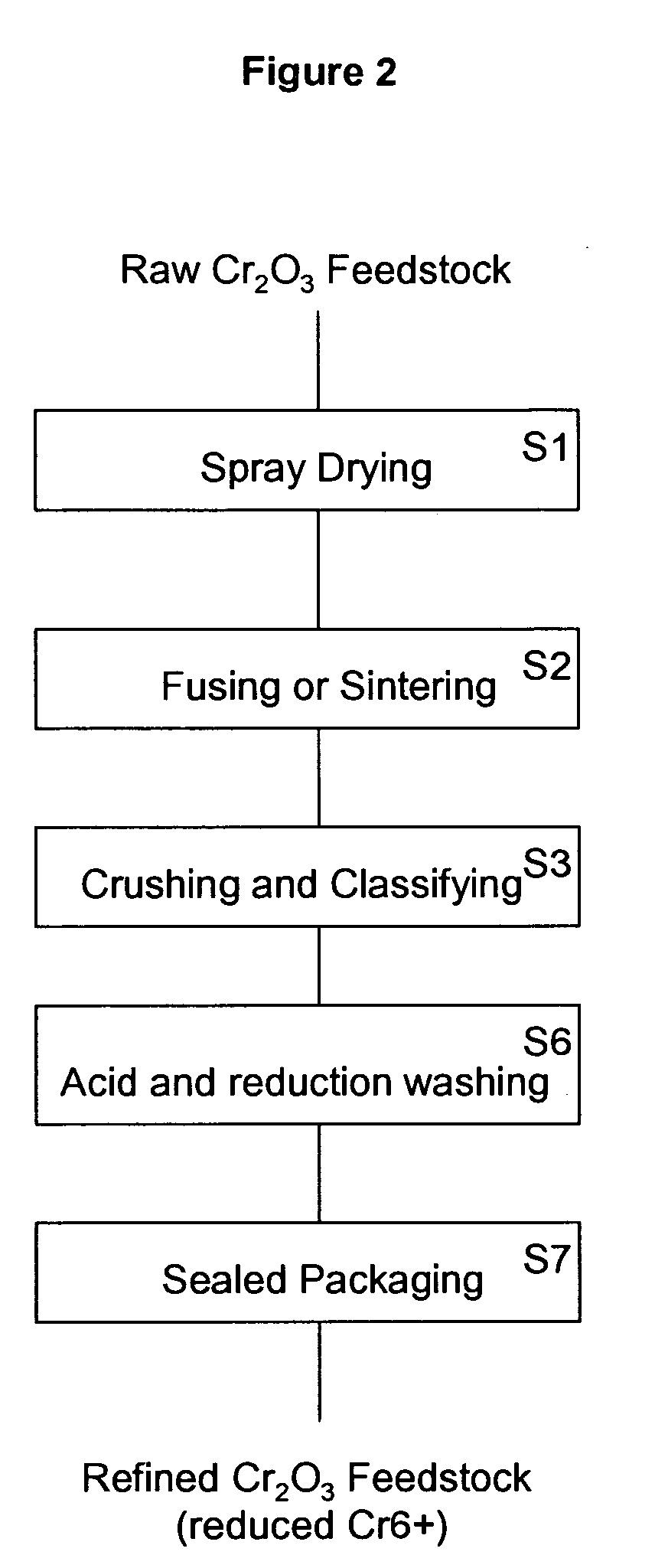
Chromium oxide powder having a reduced level of hexavalent chromium and a method of making the powder
InactiveUS20090162273A1Lower Level RequirementsImprove securityChromium oxides/hydratesMoistureHeat treating
A refined chromium oxide powder having a reduced level of hexavalent chromium. The refined powder may be produced by acid and reduction washing or by heat treatment to reduce the hexavalent chromium to a biocompatible level. The powder is then packaged to limit oxidation and absorption of moisture.
Owner:HOWMEDICA OSTEONICS CORP
Oxidatively regenerable adsorbents for sulfur removal
InactiveUS20090065400A1Increase capacityHigh selectivityRare earth metal oxides/hydroxidesGold compoundsSorbentSulfur
Compositions and processes are disclosed for removing sulfur and sulfur compounds from hydrocarbon fuel feedstocks. The feedstock is contacted with a regenerable sorbent such as a compound of the formula TixCeyO2 where 0<x / y≦1 and where 0<x≦1 and 0<y≦1 capable of selectively adsorbing sulfur compounds present in the hydrocarbon feedstock at about 0° C. to about 100° C. such as at about 25° C.
Owner:PENN STATE RES FOUND
Process for simultaneous recovery of chromium and iron from chromite ore processing residue
InactiveUS7220394B2Safe disposalSimple eco-friendly and cost-effectiveSolvent extractionMolybdeum compoundsIron saltsChromate salt
The present invention relates to a process for simultaneous recovery of chromium and iron from Chromite Ore Processing Residue (COPR) and more particularly, the present invention relates to an economical and environment-friendly process for recovering chromium as a chromate salt and iron as an iron salt from non-leachable Chromite Ore Processing Residue and avoids landfilling of toxic metals.
Owner:COUNCIL OF SCI & IND RES
Method of preparing transition metal oxide nano-particles
ActiveCN101830496AEasy to manufactureEasy to handleNanostructure manufactureTantalum compoundsAlcoholMetallate
Provided is a method for preparing transition metal oxide nano-particles from a transition metal as a reactant. The method includes dissolving the transition metal into aqueous hydrogen peroxide to provide peroxi-metallate solution, and then adding a reactive solution containing an alcohol, water and an acid thereto to perform hydrothermal reaction. More particularly, the method for preparing transition metal oxide particles includes: dissolving transition metal powder as a reactant into aqueous hydrogen peroxide to provide a peroxi-metallate solution with a molar concentration of transition metal of 0.001-0.2 M; adding a reactive solution containing an alcohol, water and an acid to the peroxi-metallate solution to provide a mixed solution; and subjecting the mixed solution to hydrothermal reaction to provide transition metal oxide nano-particles.
Owner:THE IND & ACADEMIC COOPERATION & CHUNGNAM NAT UNIV
Method for synthesizing high specific surface area ordered mesoporous metal oxide by using hard template agent
InactiveCN101214928ANarrow pore size distributionReduce manufacturing costOxide/hydroxide preparationRare earth metal compoundsAlcoholMesoporous material
A synthesis method of order mesoporous metal oxide with high specific surface area by hard template agent belongs to the filed of the preparation of solid mesoporous materials. The prior art is difficult to control the problems of the hydrolysis rate of metal salt, easy collapse and the poor universality of channels when templates are removed by calcination, etc. In the invention, firstly, hard template agent cubic-phase mesoporous silicon oxide powder is synthetized; the silicon oxide powder is added into 50 to 95wt percent of ethyl alcohol solution of metal salt with the concentration of 0.12 to 0.40 mol / L to form mixed solution; after 90 to 120 min of ultrasonic dispersion, the product is mixed again, vaporized under the temperature of 40 DEG C to 50 DEG C and dried; and then the product is heated to 400 DEG C to 850 DEG C in atmosphere at 1 DEG C / min, and kept for 4 hours under the temperature to obtain the mesoporous oxide precursor powder; the mol ratio of the cubic-phase mesoporous silicon oxide powder and the metal salt is 7-14; 10wt percent of HF solution is used to wash, so as to remove the cubic-phase mesoporous silicon oxide template agent, and the product is dried under 60 DEG C to 70 DEG C for 20 to 24 hours. The mesoporous metal oxide of the invention is suitable to be adsorbent, catalyst, carrier, etc., and is applicable to dyes, magnetic, photoelectric materials, etc.
Owner:BEIJING UNIV OF TECH
Method for directly preparing pigment grade chromium hemitrioxide green by using chromic hydroxide
The invention discloses a method for preparing pigment-grade chromium oxide green directly from chromic hydroxide. The method comprises the steps of grinding and mixing chromic hydroxide and a certain quantity of additives, activating and sintering a mixture for 0.5 to 3 hours at a temperature between 800 and 1,100 DEG C, washing, drying and grading the mixture so as to obtain the pigment-grade chromium oxide green, wherein the chromic hydroxide can be obtained from the prior industrial production and various types of waste (waste residue and wastewater), or can be obtained by a low-temperature hydrogen reduction method in a patent (application number: 200510089010.8), and the additives are silicon compounds, titanium compounds, boron compounds, phosphorus compounds, aluminum compounds, barium compounds, zinc compounds and other compounds, such as SiO2, K2SiO3, Na2SiO3, TiO2, K2TiO3, B2O3, H2BO3, H3PO4, P2O3, Al2O3, Al(OH)3, Al2(SO4)3, AlCl3, Al(NO3)3, BaO, Ba(OH)2, BaSO4, BaCl2, Ba(NO3)2, ZnO, ZnCl2, ZnSO4, Zn(NO)3, Sb2O3, NiO, Ni(OH)2, CoO, Co(OH)2 and other substances. After the chromic hydroxide is activated and sintered at a temperature between 700 and 1,300 DEG C in an air atmosphere, the alkali-metal content of obtained chromium oxide is less than 0.3 percent, and cannot influence the properties of the pigment-grade chromium oxide green.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
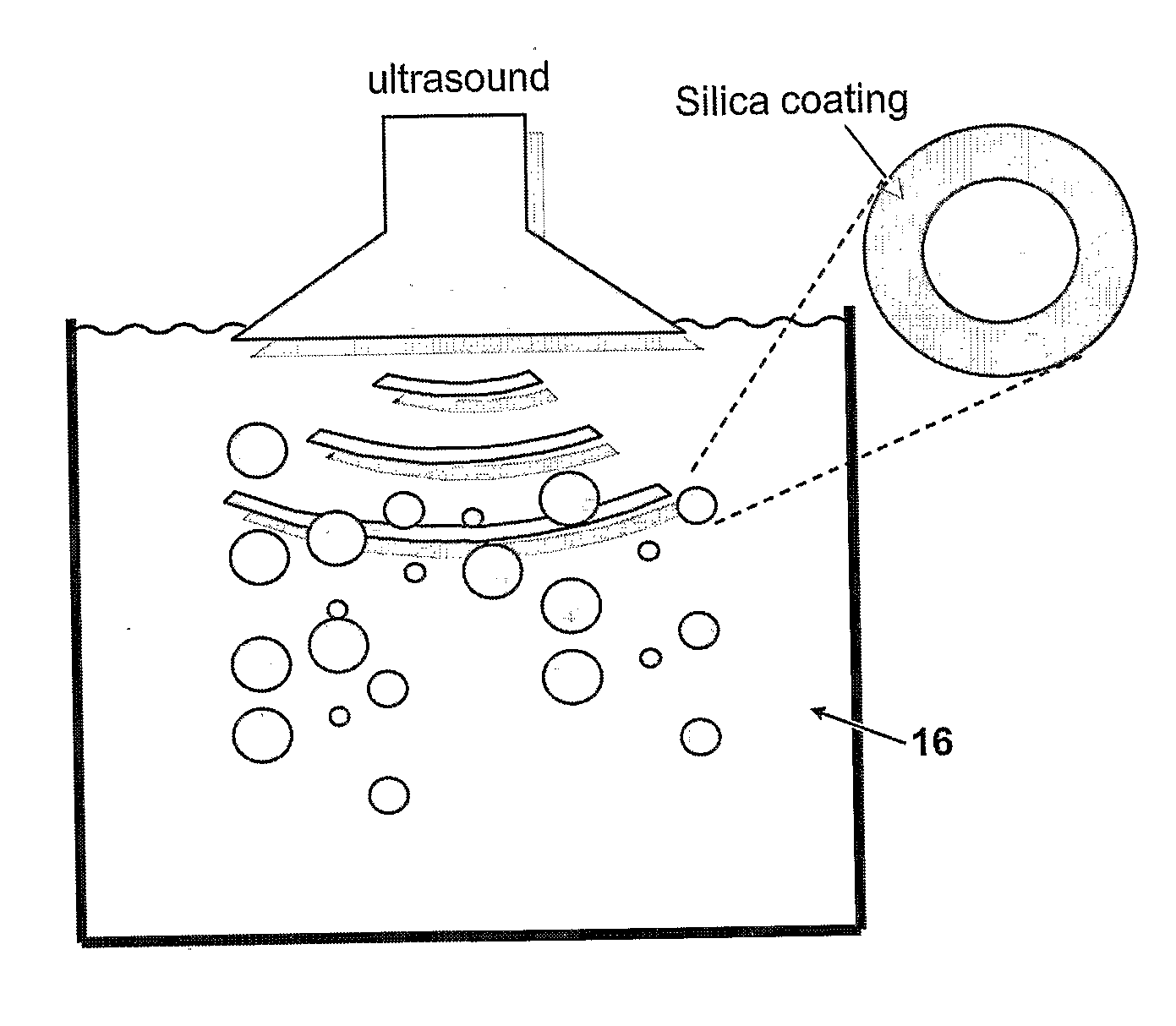
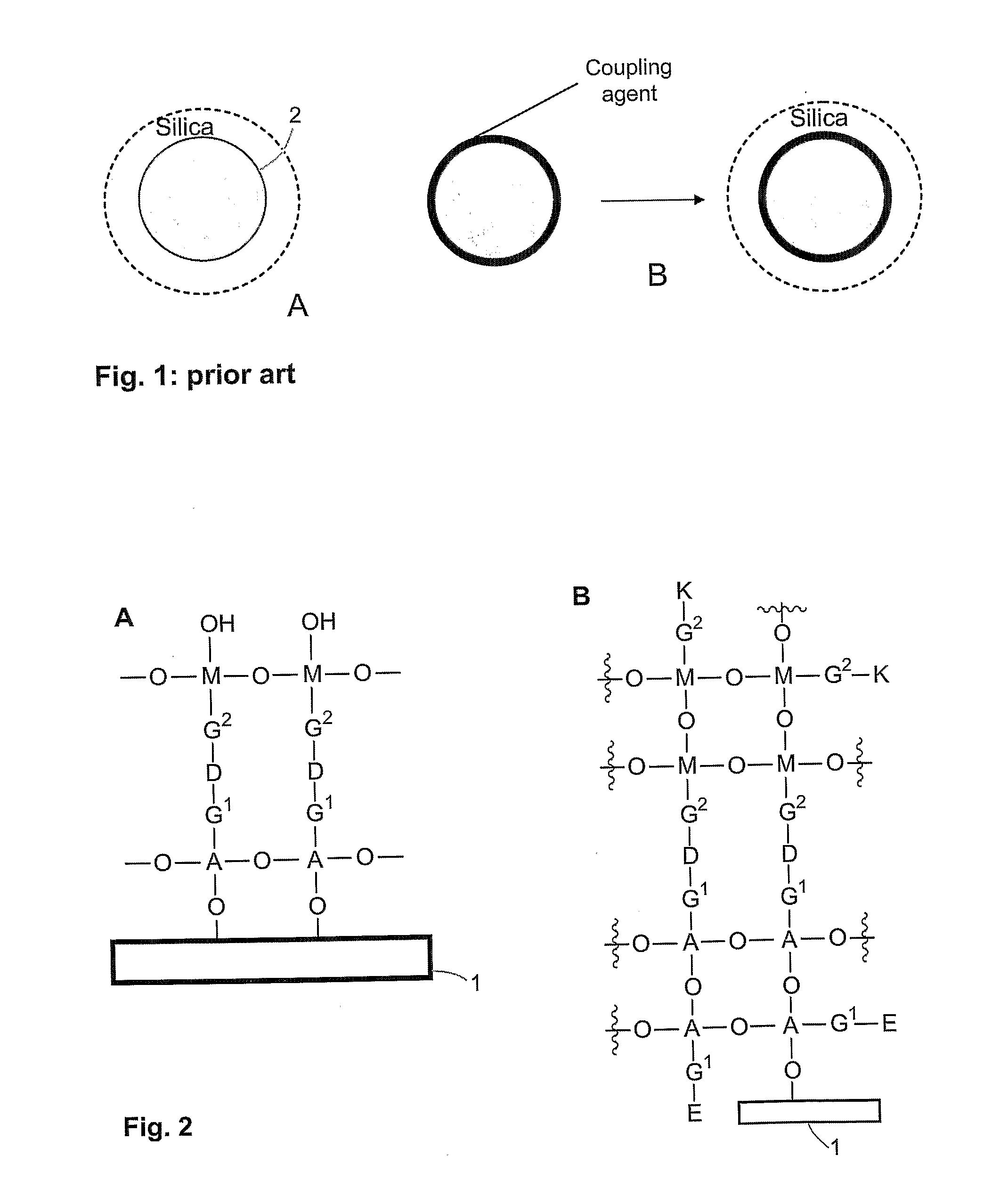
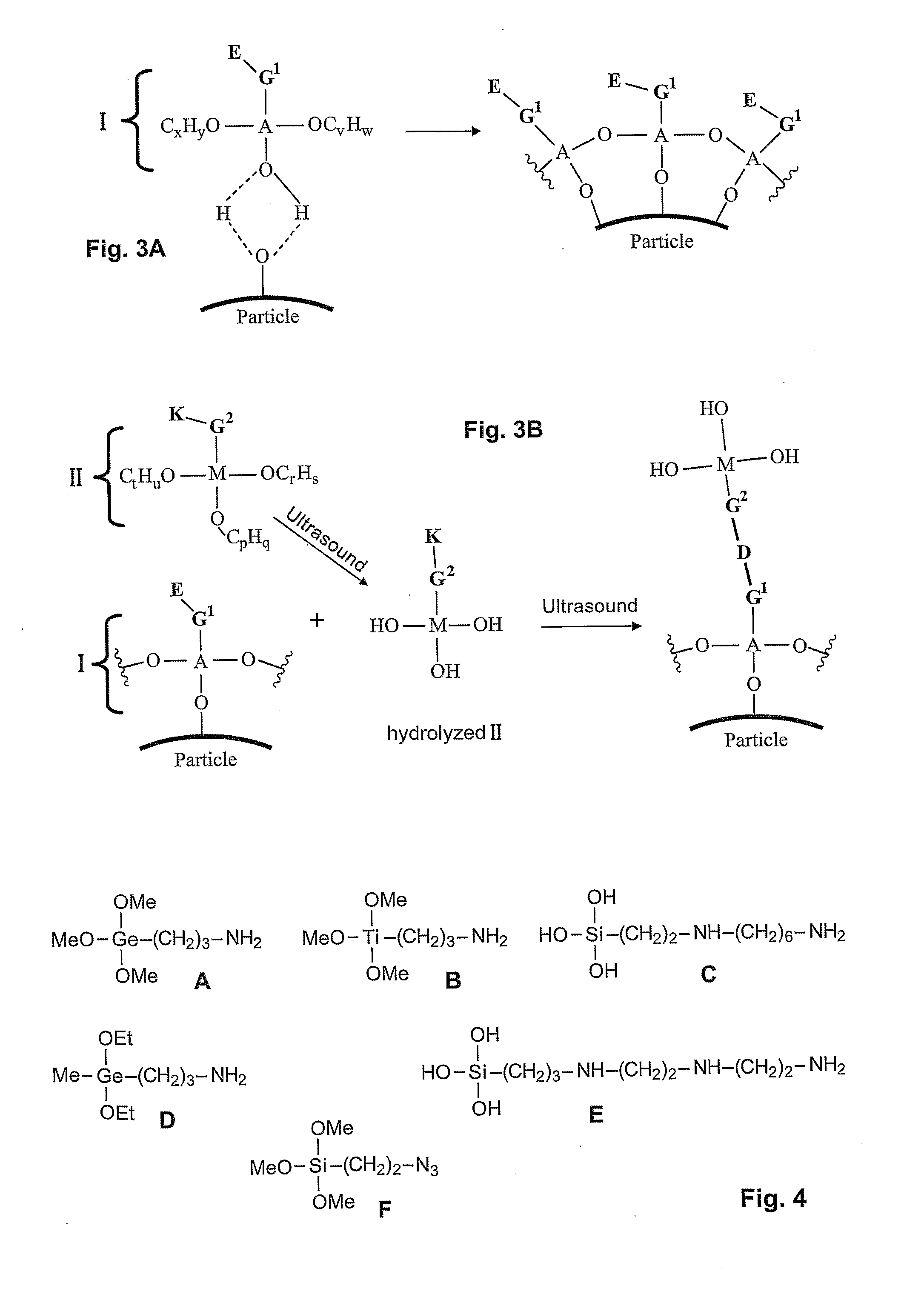
Method of coating a particle
Disclosed is a method of coating a particle, the method comprising contacting (i) a particle, wherein the surface of the particle comprises functional groups suitable to undergo a coupling reaction with at least a hydroxyl group, a halogen group or an alkoxy group, with (ii) molecules of formula (Ia) and / or (Ib): (Ia) or (Ib) wherein A is a metal or a metalloid, R1 and (in formula (Ia)) R2 are halogen or an alkoxy group and R20 is OH, halogen or an alkoxy group. G1 is an aliphatic; cycloaliphatic, aromatic, arylaliphatic or arylcycloaliphatic spacer and E is a functional group. Molecules of the compound of general formula (Ia) and / or (Ib) are immobilized on the surface of the particle by a coupling reaction between functional groups on the surface of the particle and moieties R20.
Owner:AGENCY FOR SCI TECH & RES
Method for preparing high specific surface area chromium series fluorating catalyst former body chromic oxide
InactiveCN101417816ALarge specific surface areaEasy to makeChromium oxides/hydratesMetal/metal-oxides/metal-hydroxide catalystsGas phasePolyethylene glycol
The invention relates to a preparation method for precursor chromic oxide of a chromic fluorination catalyst with high specific surface area, which is characterized by comprises the following steps of: completely dissolving an organic addition agent which can be a polyglycol-series PEG1000-6000, or nonionic surface active agents Tween40, Tween80, or a cation surface active agent dodecyl cetyl trimethyl ammonium bromide in soluble chromic salt solution, controlling the weight ratio of the organic addition agent and the soluble chromic salt to be 0.1: 1 to 0.8: 1, and precipitating by a precipitator under stirring condition, filtering, washing and drying. By adding the organic additive, the method effectively improves the specific surface area of Cr2O3 question mark nH2O (n is less than 3 and more than 0) and has simple and easily operated preparation process and good experimental reproducibility. The prepared compound can be used for producing the fluorination catalyst of HFCs which is the substitute of Freon in a gas phase method after high temperature roasting and activation.
Owner:江苏梅兰化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com