Preparation method of Sacubitril intermediate
An intermediate and weight ratio technology, applied in the field of compound preparation, can solve the problems of difficult to obtain intermediates, cumbersome routes, large amount of auxiliary reagents, etc., and achieve the effects of short method steps, increased reaction yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
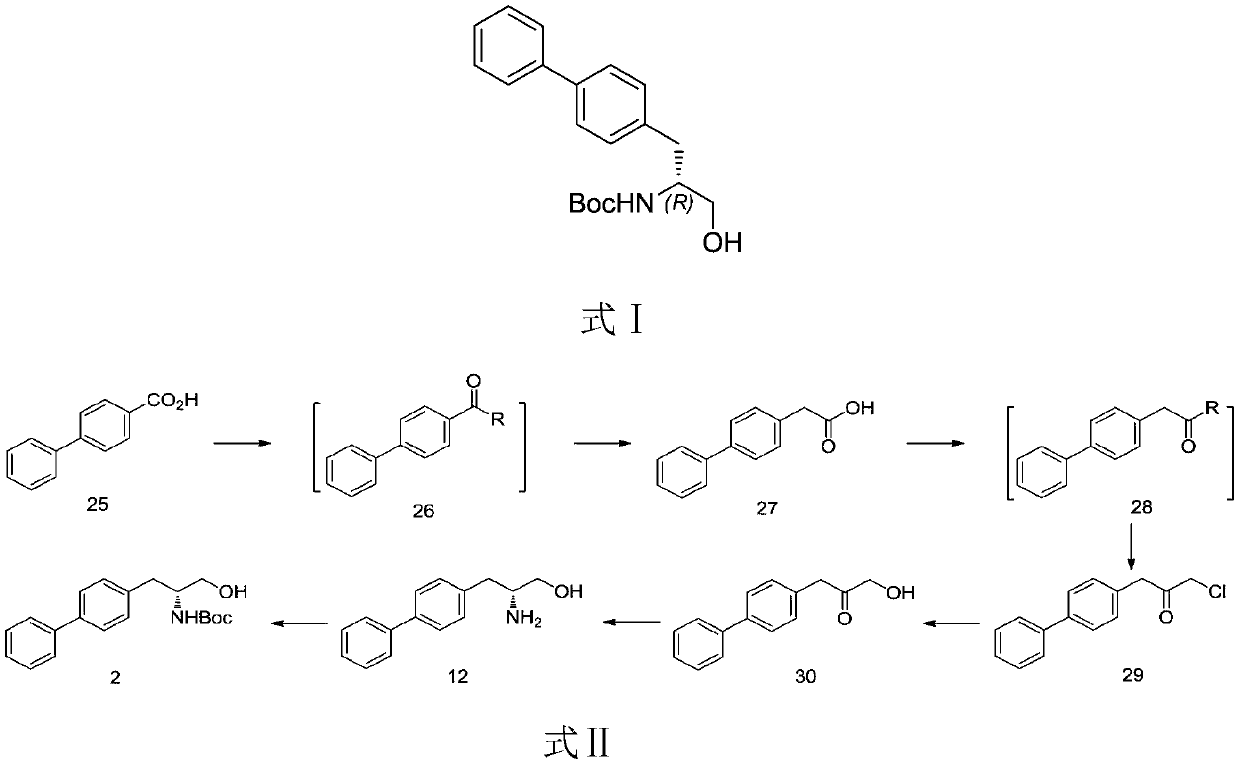
[0040] Example 1: In the synthetic route shown in Formula 7, the acyl halide reagent is thionyl chloride, and R is a chlorine atom; the base used to convert compound 29 into compound 30 is sodium hydroxide.
[0041] Preparation of compound 26
[0042] Compound 25 (19.8 g, 0.1 mol) was added to a 500 mL three-necked flask, 100 mL of toluene was added, and thionyl chloride (14.3 g, 0.12 mol) was added dropwise under cooling in an ice-water bath. After dripping, the cold bath was removed, the temperature was raised to reflux for 5 hours, cooled to room temperature, and stored for later use.
[0043] Preparation of compound 27
[0044] Add 250mL of methyl tert-butyl ether and 90mL of diethylene glycol dimethyl ether into a 500mL three-necked flask, add methylnitrosourea (30g, 0.29mol) under cooling in an ice-water bath, stir for 10 minutes, and store at low temperature until use. Methyl nitrosourea solution and 30% potassium hydroxide solution (50 g, 0.89 mol) were prepared in ...
Embodiment 2
[0057] Example 2: In the synthetic route shown in formula 7, the acyl halide reagent is oxalyl chloride, R chlorine atom; the base used to convert compound 29 into compound 30 is sodium formate.
[0058] Preparation of compound 26
[0059] Compound 25 (19.8 g, 0.1 mol) was added to a 500 mL three-necked flask, 100 mL of dichloromethane was added, and oxalyl chloride (13.7 g, 0.12 mol) was added dropwise under cooling in an ice-water bath. After dripping, the cold bath was removed, raised to room temperature and reacted for 4 hours, cooled to room temperature, and stored for later use.
[0060] Preparation of compound 27
[0061] Add 250mL of methyl tert-butyl ether and 90mL of diethylene glycol dimethyl ether into a 500mL three-necked flask, add methylnitrosourea (30g, 0.29mol) under cooling in an ice-water bath, stir for 10 minutes, and store at low temperature until use. Methyl nitrosourea solution and 30% potassium hydroxide solution (50 g, 0.89 mol) were prepared in a m...
Embodiment 3
[0074] Example 3: In the synthetic route shown in Formula 7, the acyl halide reagent is phosphorus oxychloride, and R is a chlorine atom; the base used to convert compound 29 into compound 30 is potassium hydroxide.
[0075] Preparation of compound 26
[0076] Compound 25 (19.8 g, 0.1 mol) was added to a 500 mL three-necked flask, 100 mL of dichloromethane was added, and phosphorus oxychloride (18.4 g, 0.12 mol) was added dropwise under cooling in an ice-water bath. After dripping, the cold bath was removed, raised to room temperature and reacted for 2 hours, and stored for later use.
[0077] Preparation of compound 27
[0078] Add 250mL of methyl tert-butyl ether and 90mL of diethylene glycol dimethyl ether into a 500mL three-necked flask, add methylnitrosourea (30g, 0.29mol) under cooling in an ice-water bath, stir for 10 minutes, and store at low temperature until use. Methyl nitrosourea solution and 30% potassium hydroxide solution (50 g, 0.89 mol) were prepared in a m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com