Preparation method and application of sacubitril intermediate
A technology for sacubitril and intermediates, which is applied in the field of medicine and chemical industry, can solve the problems of repeated protection of carboxyl groups and amino groups, numerous reaction steps, unfavorable industrial production and the like, and achieves the effects of easy availability of raw materials, cheap raw materials and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
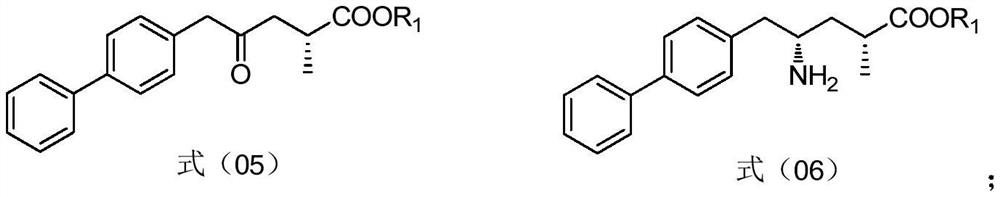
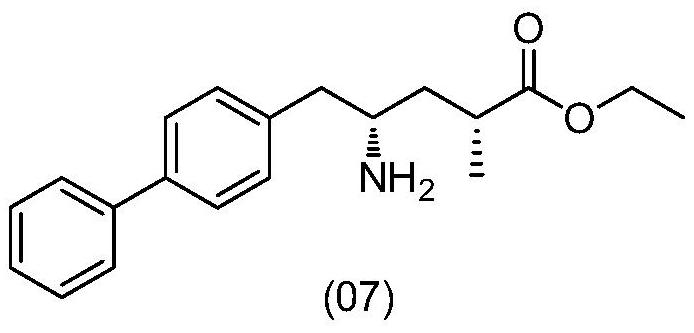
Image
Examples
Embodiment
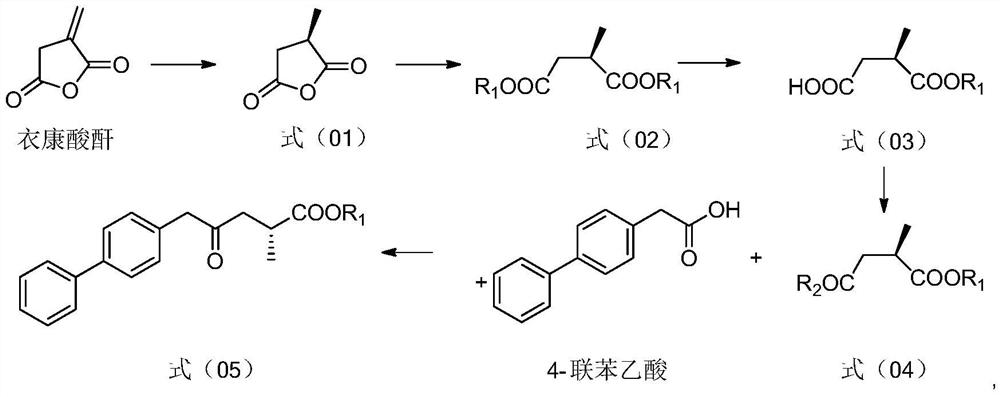
[0041] 1. Preparation of R-methylsuccinic anhydride (1)
[0042]
[0043] Add itaconic anhydride (50g, 446.4mmol) and ethyl acetate (250ml) in a 500ml hydrogenation kettle, blow with argon for 10min, add (S,S)-Me-Duphos (136.6mg, 0.45mmol) and paracyme Hydrocarbon ruthenium dichloride (275.6mg, 0.45mmol), pressurized hydrogen to 5MPa, reacted at 35°C for 24h, TLC detected that the reaction was complete, concentrated to 100ml, a white solid was precipitated, filtered and dried to obtain a white solid 1( 49.3g%, yield 96.9%).
[0044] 1 HNMR (500MHz, CDCl3) δ3.25–3.14 (m, 2H), 2.68–2.59 (m, 1H), 1.45 (d, J=9.0Hz, 3H). MS (ESI) m / z: 115.1 [M+ H]+.
[0045] 2. Synthesis of ethyl R-methylsuccinate (2)
[0046]
[0047] Add R-methylsuccinic anhydride (49.3g, 432.5mmol) and ethanol (200ml) into a 500ml single-necked bottle, add 5ml of concentrated sulfuric acid, heat to reflux for 24h, and TLC detects that the reaction is complete. The solvent was spin-dried, ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com