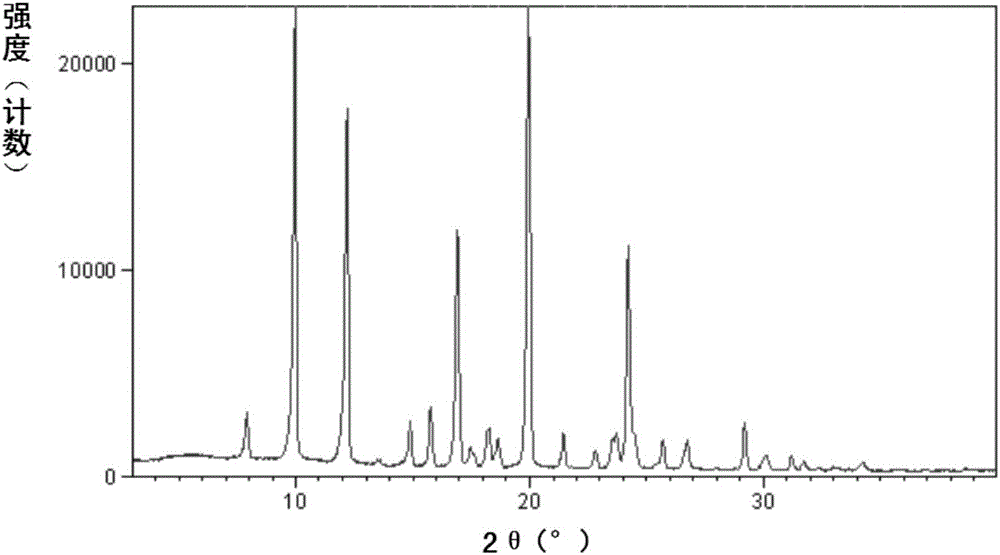
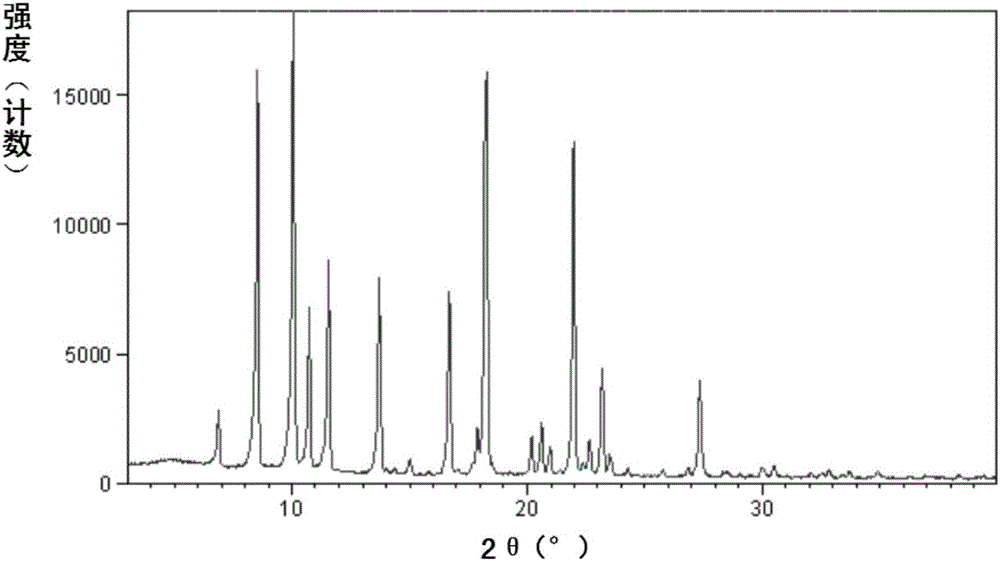
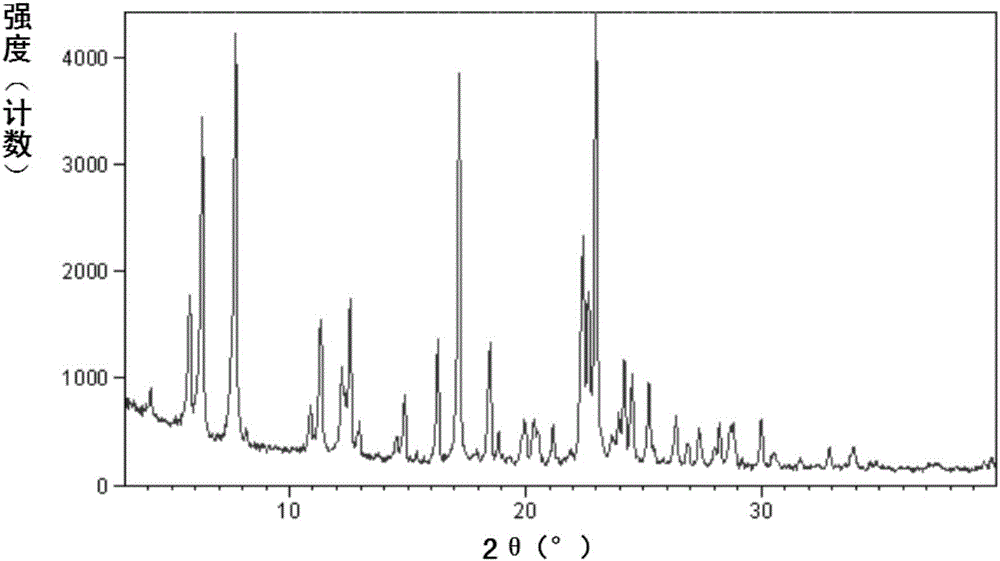
Improved preparation method of sacubitril intermediate
A technology of sacubitril and compound, which is applied in the field of neutral endopeptidase inhibitors, and can solve the problems of high hydrogen pressure, high price, and difficulty in obtaining chiral catalysts.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 Preparation of (2R, 4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpentanoic acid (III)
[0082]
[0083] At 35~40°C, (E)-(R)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpent-2-enoic acid (IV) 50.0 g (0.131mmol, 1eq), 10% palladium / carbon 2.5g and tetrahydrofuran 500ml were mixed in a hydrogenation reactor. After the replacement with hydrogen, 1.5-2 atmospheres of hydrogen was introduced, and the reaction was stirred for about 3 hours. Filtrate and concentrate the filtrate to obtain crude product of (2R,4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpentanoic acid (compound of formula III), chiral HPLC: 85.6% .
[0084] Stir and mix about 20 g of the crude product of (2R,4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpentanoic acid (compound of formula III) with 60 ml of acetone, Heated to reflux, and 120ml of n-heptane was added dropwise. After the addition, it was naturally lowered to room temperature, stirred an...
Embodiment 2
[0092] Example 2 Preparation of (2R, 4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpentanoic acid diisopropylamine salt (XII)
[0093]
[0094] At 30~35°C, (E)-(R)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpent-2-enoic acid (IV) 50.0 g (0.131mol, 1eq), 14.6g (0.144mol, 1.1eq) of diisopropylamine, 5.0g of 10% Pd / C and 250ml of ethanol were mixed in a hydrogenation reactor. After hydrogen replacement, 2-2.5 atmospheres of hydrogen was added, and the reaction was stirred for about 3 hours. Filtration, and the filtrate was concentrated to obtain (2R,4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpentanoic acid diisopropylamine salt (compound of formula XII) crude product, chiral HPLC: 90.8%.
[0095] Mix the crude product of (2R,4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpentanoic acid diisopropylamine salt (compound of formula XII) with 250ml of acetone, and stir . Heated to reflux, and 750ml of n-heptane was added dropwise. A...
Embodiment 3
[0096] Example 3 (2R, 4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpentanoic acid (S)-(-)-α-phenethylamine salt ( XIII) Preparation
[0097]
[0098] At 25~30°C, add (E)-(R)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpent-2-enoic acid (IV) 50.00g (0.131mol, 1eq), (S)-(-)-α-phenethylamine 12.7g (0.105mmol, 0.8eq), 5% Pd / C 10.0g and methyl tetrahydrofuran 600ml were mixed and stirred in a hydrogenation reactor. After hydrogen replacement, 2.5 to 3 atmospheres of hydrogen was added, and the reaction was stirred for about 3 hours. Filtration, and the filtrate was concentrated to obtain (2R,4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methylpentanoic acid (S)-(-)-α-phenylethylamine Salt (compound of formula XIII), chiral HPLC: 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com