Method for measuring concentrations of sacubitril, desethyl sacubitril and valsartan in human plasma
A technology of ethyl sacubitril and sacubitril, which is applied in the field of detecting drug concentration in human plasma, can solve problems such as lack, and achieve the effects of good peak shape, high response signal and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1: Determination of sacubitril, desethylsacubitril and valsartan in plasma samples
[0102] Liquid phase conditions: mobile phase: acetonitrile-0.1% formic acid aqueous solution (v / v, 60:40), flow rate: 0.4 ml / min, column temperature and sample chamber temperature are both 20°C.
[0103]Mass spectrometry conditions: TRIPLE QUAD 5500 triple quadrupole tandem mass spectrometer of AB Company (equipped with electrospray ionization ESI source, Analyst 1.6.3 software). Electrospray ionization (ESI), positive ion mode, multiple reaction detection mode (MRM), spray voltage 5500V, ion source temperature 550°C, curtain gas: 35psi, atomizing gas: 45psi, auxiliary gas: 40psi. The ion pairs, residence time, etc. are as follows:
[0104] Table 1. Mass Spectrometry Information for Analytes and Internal Standards
[0105]
[0106] Pretreatment of plasma samples: Precisely draw 100 μL of plasma sample (the fourth concentration point of the standard curve), put it into a 2ml...
Embodiment 2
[0108] Example 2: Influence experiment of different diluents
[0109] Detect according to the method of Example 1. When other experimental conditions are the same, use different diluents: pure water and 50% methanol water to dilute the 100 μL supernatant obtained from the pretreatment of the sample, respectively inject the sample for analysis, and record the chromatogram. See Figure 2-1 ~ Figure 3-3 . It can be seen from the chromatogram that when the diluent is the 80% methanol water used in Example 1, the peak shape is the best.
Embodiment 3
[0110] Example 3: Methodological Validation—Exclusive Experiment
[0111] Take blank plasma (no internal standard was added during the sample pretreatment process), blank plasma, the fourth concentration plasma sample of the standard curve, and the plasma collected 1.75 hours after fasting administration. figure, see Figure 4-1-A ~ Figure 7-3-B .
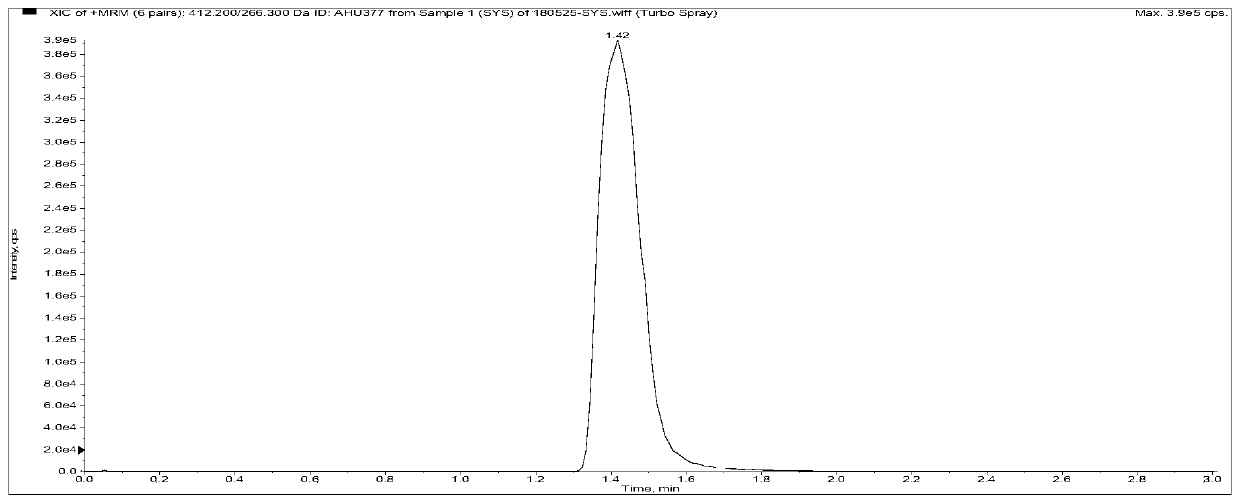
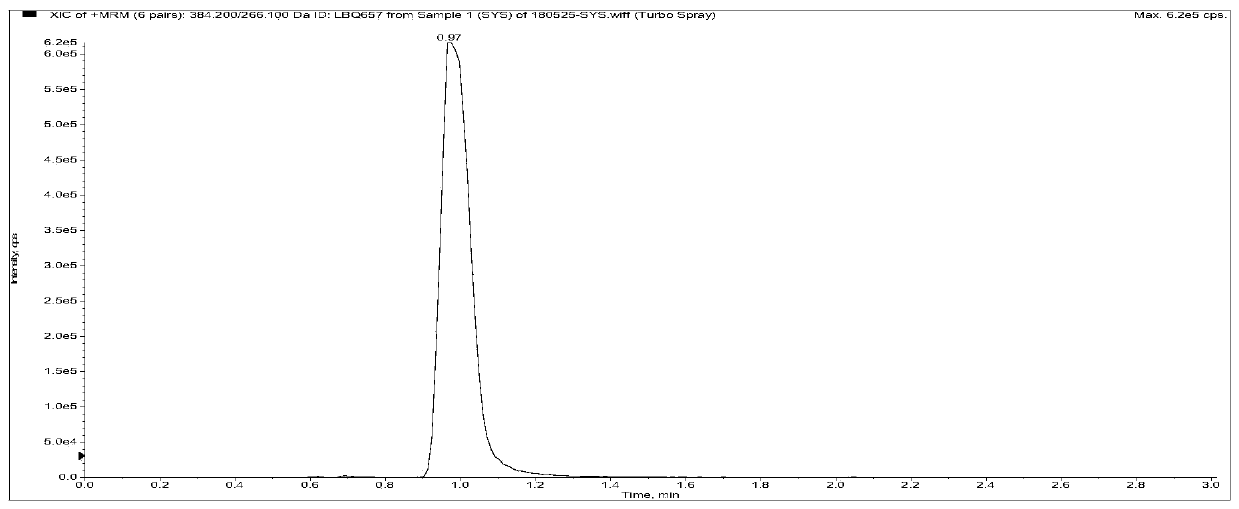
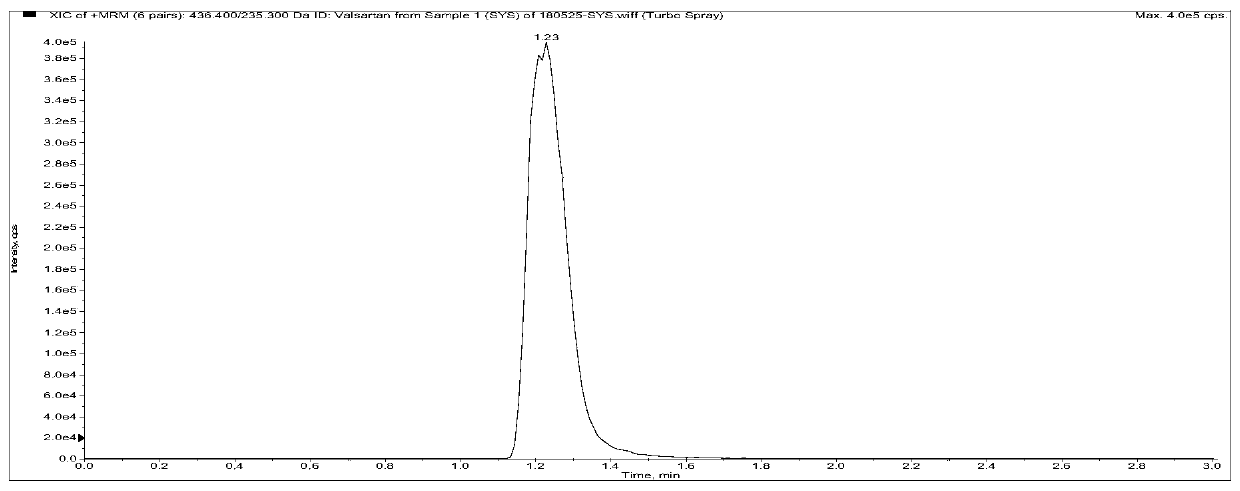
[0112] Among them, the retention time of AHU377 was 1.37min, the retention time of LBQ657 was 0.97min, the retention time of Valsartan was 1.18min, the peak appeared within 3min, and the impurities in the plasma did not interfere with the determination (the blank plasma Figure 5-1-A In the chromatogram, the analyte AHU377 exists in the chromatogram because the internal standard is impure, but the low content does not affect the subsequent detection of AHU377), and the specificity of this method is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com