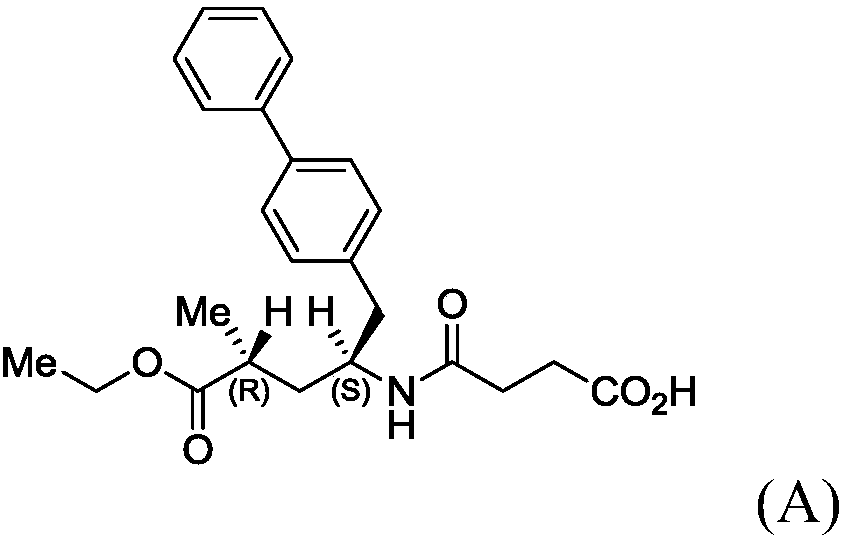
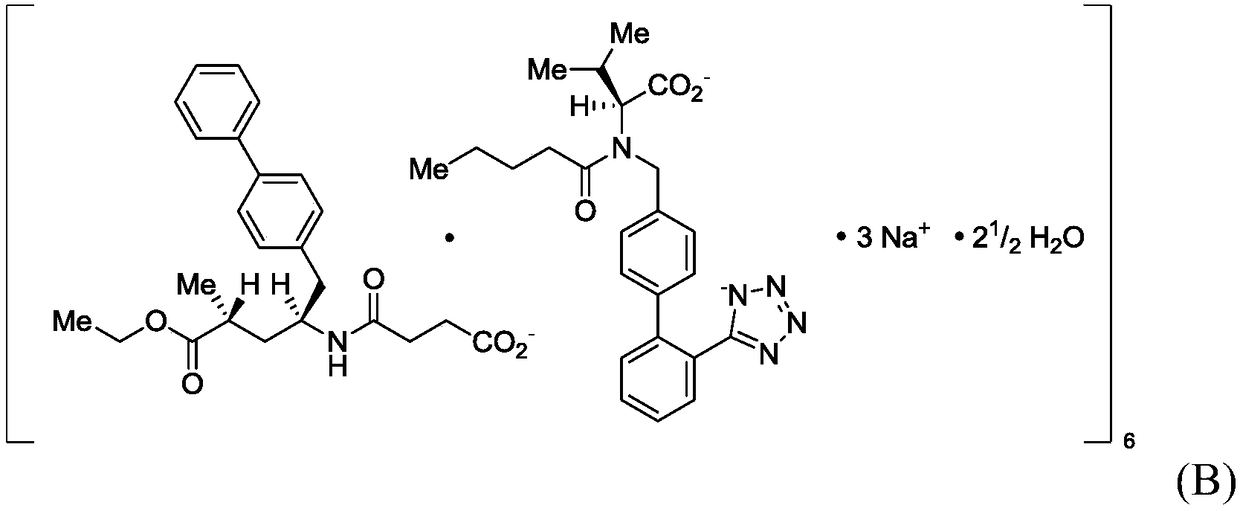
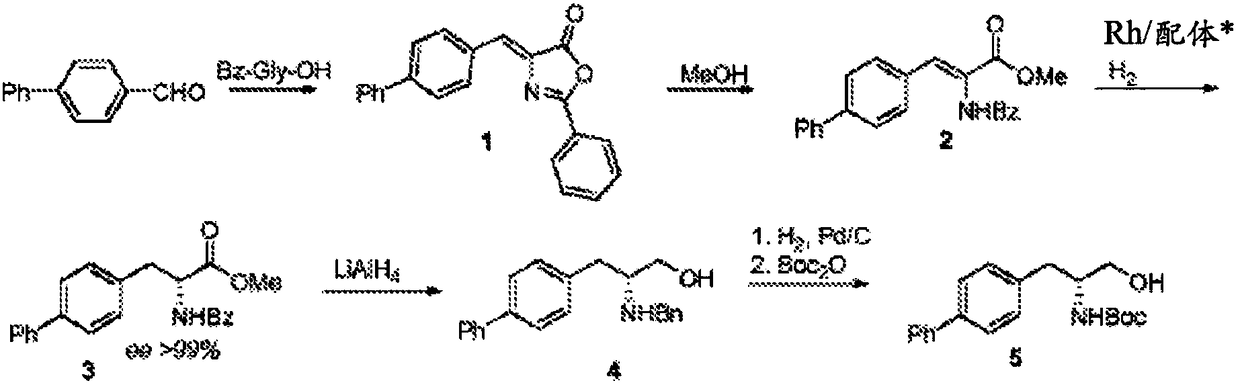
Novel process for early sacubitril intermediates
A compound and nitrogen protection technology, applied in the direction of organic chemical methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of potential danger, expensive catalyst stereoselectivity, etc., and achieve high stereoselectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0135] Where necessary, the following sections describe in more detail individual method steps as set forth above or as delineated in the claims.
[0136] In a first aspect, the present invention relates to a compound of formula (II) or a salt thereof
[0137]
[0138] wherein R1 is a hydrogen or nitrogen protecting group.
[0139] In one embodiment thereof, the compound has formula (II-a) or a salt thereof
[0140]
[0141] wherein R1 is a hydrogen or nitrogen protecting group.
[0142] In one embodiment, R1 is hydrogen.
[0143] In another embodiment, R1 is a nitrogen protecting group selected from: C 1 -C 6 -Alkyl, which is unsubstituted or replaced by three-C 1 -C 6 -Alkylsilyl C 1 -C 7 -alkoxy monosubstituted, disubstituted, or trisubstituted; C 6 -C 10 -aryl; or a heterocyclic group having 5 to 14 ring atoms and 1 to 4 ring atoms independently selected from N, O, S, S(O) or S(O) 2 A monocyclic, bicyclic or tricyclic ring system of heteroatoms; wherein th...
example 3
[0310] Example 3: (R)-tert-butyl (1-([1,1'-biphenyl]-4-yl)-3-hydroxypropan-2-yl)carbamate 6 manufacturing
[0311] 10% Pd / C (type 10R39, Johnson Matthey; 40% dry weight loading, 30 mg dry weight, moisture corrected to about 50%) and (S)-tert-butyl (1-([1,1 A suspension of '-biphenyl]-4-yl)-3-hydroxy-1-oxopropan-2-yl)carbamate 9 in ethyl acetate (3 mL) was heated at 25 °C under hydrogen (3 bar pressure) for 18 h. Product 6 was isolated by filtration and purified by chromatography if necessary.
[0312] 1 H-NMR (400MHz, DMSO-d6): δ2.30(d,4H),3.14(m,2H),3.73(s,3H),4.39(t,1H),7.32(m,2H),7.38( m,1H),7.44-7.52(m,2H),7.63-7.71(m,4H),8.41(br.s,3H); MS(ES-API): positive ion mode 256.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com