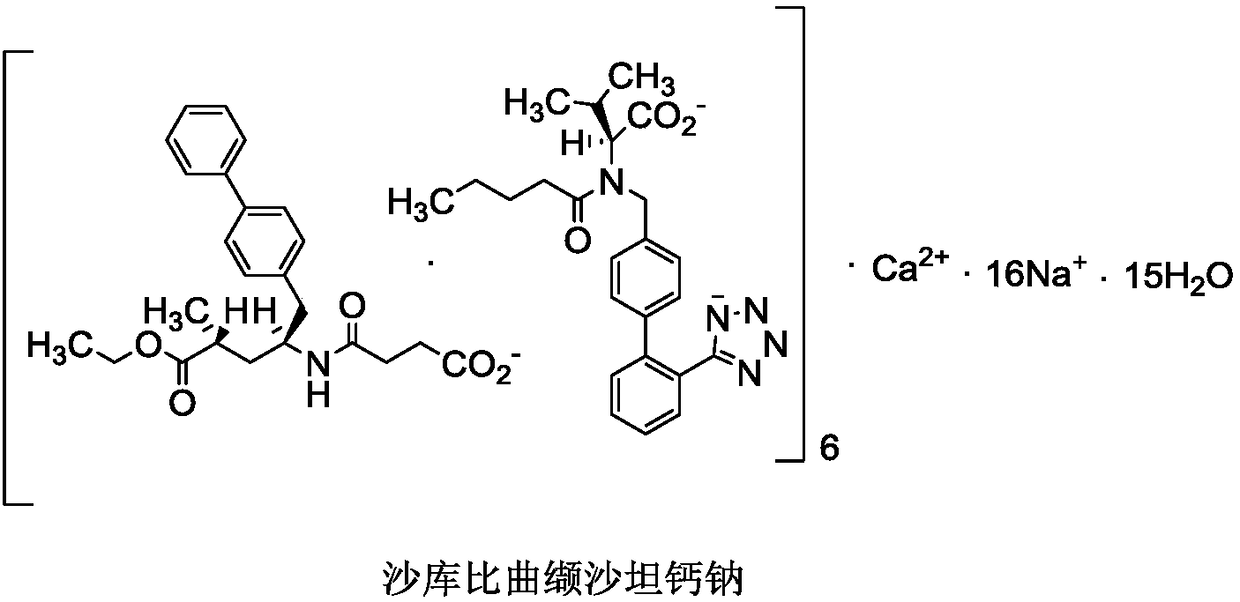
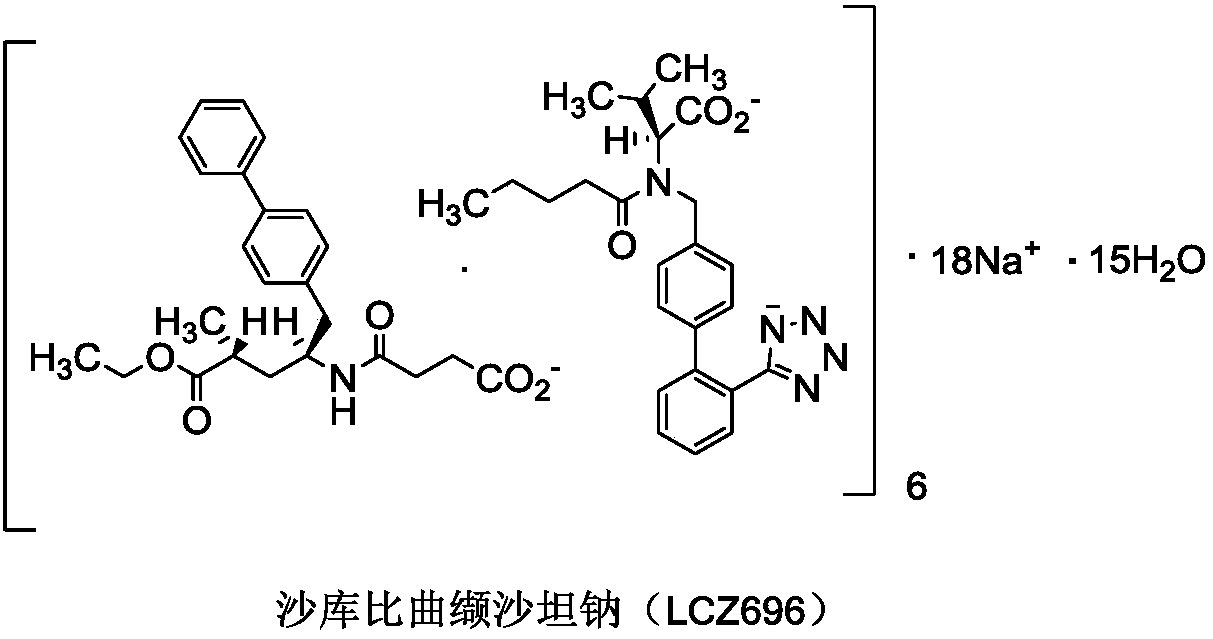
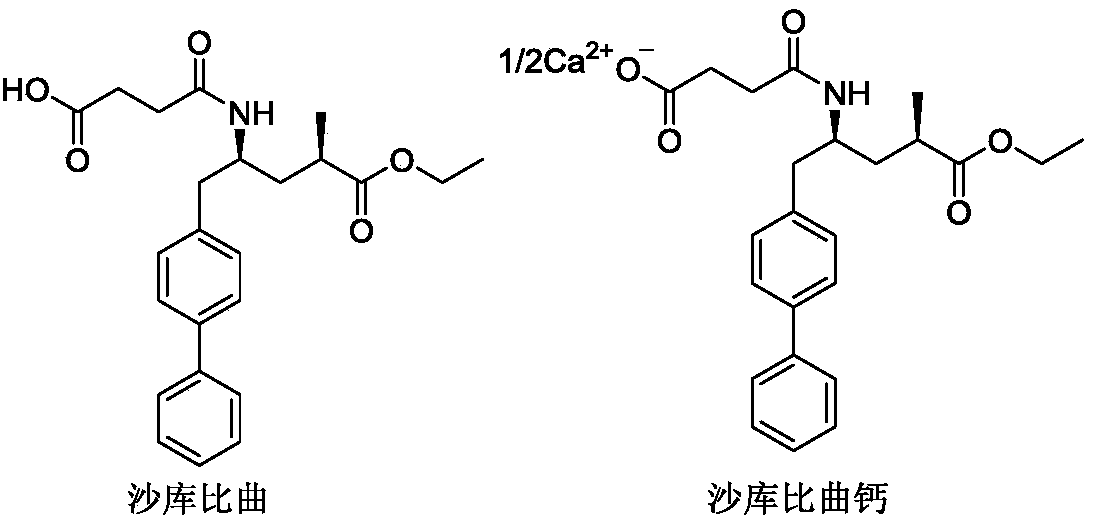
Preparation method of sacubitril-valsartan compound and/or eutectic key intermediate sacubitril calcium
A technology of sacubitril and sartan calcium sodium, which is applied in the preparation of pharmaceutical intermediates, and the preparation field of sacubitril-valsartan complex and/or co-crystal key intermediate sacubitril calcium, can solve the problem of Unfavorable to industrialized production, poor stereoselectivity, long reaction steps, etc., to achieve the effects of being beneficial to industrialized production, low cost, and few processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Compound II ((2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methyl-pentanoic acid ) preparation
[0037] Add (R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methyl-pentane to the hydrogenation vessel under stirring -2-enoic acid (compound III) (75g, 0.20mol), 10% palladium on carbon (Pd / C) (3.0g) and 750ml of ethanol, hydrogen was passed through and kept under pressure (1.0MPa, 25°C) for 20 hours. Filtrate, concentrate to dryness, add an appropriate proportion of isopropyl acetate / petroleum ether for recrystallization, and obtain a white solid (compound II) after drying, the specific data is shown in the table below. 1 H NMR (DMSO-d 6 )δ12.01(s,1H),7.63(d,2H,J=7.6Hz),7.56(d,2H,J=8.0Hz),7.47-7.43(m,2H),7.35(d,1H,J =7.2Hz),7.24(d,2H,J=8.0Hz),6.73(d,1H,J=8.8Hz),3.67-3.66(m,1H),2.68(d,2H,J=6.8Hz), 2.45-2.42(m,1H),1.78-1.71(m,1H),1.32(s,9H),1.40-1.32(m,1H),1.06(d,3H,J=6.8Hz).
Embodiment 2
[0040] Example 2 Preparation of Compound I ((2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-amino-2-methyl-pentanoic hydrochloride)
[0041] Add (2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methyl-pentane to the reaction flask under stirring Acid (compound II) (45g, 0.12mol), ethanol 225ml, thionyl chloride (69.3g, 0.58mol) was added dropwise at 5°C, after the addition was completed, the temperature was raised to 40°C to react for 4 hours. Concentrate to dryness, beat with petroleum ether, filter, and dry to obtain 39.5 g of off-white solid (Compound I), with a mass yield of 87.8%. 1 H NMR (DMSO-d 6 )δ8.33(s,3H),7.68-7.64(m,4H),7.49-7.45(m,2H),7.38-7.36(m,3H),4.01-3.96(m,2H),3.38-3.36( m,1H),3.12-3.08(m,1H),2.85-2.74(m,2H),1.89-1.83(m,1H),1.66-1.60(m,1H),1.09(t,3H,J=12.4 Hz), 1.07 (d, 3H, J=5.2Hz). HPLC 99.8%, isomer≤0.2%.
[0042]
Embodiment 3
[0043] Example 3 Shakubitra calcium (4-(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5 Preparation of -oxopent-2-yl)amino)-4-oxobutyrate calcium)
[0044] Add (2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-amino-2-methyl-pentane hydrochloride (compound I) into the reaction flask under stirring (36g, 0.10mol), succinic anhydride (9.7g, 0.10mol) and DMF 90ml, add an appropriate amount of calcium-containing alkali (mixture of one or more kinds) in batches at 0-10°C, after adding, heat up to 25°C for reaction 4 hours. Add water, beat, filter, and get crude product. Add an appropriate proportion of organic solvent and water to the mixed system for recrystallization, filter, and dry to obtain an off-white solid (Sacubitronic Calcium), with all isomers ≤ 0.1%, and other data are shown in the table below.
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com