New crystal of sacubitril potassium salt, and preparing method and use thereof
A technology of sacubitril and potassium salt, which can be used in the preparation of organic compounds, the preparation of carboxylic acid amides, chemical instruments and methods, etc., and can solve problems such as undisclosed crystal physical and chemical data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Preparation of Shakubiqu Potassium Salt Form B
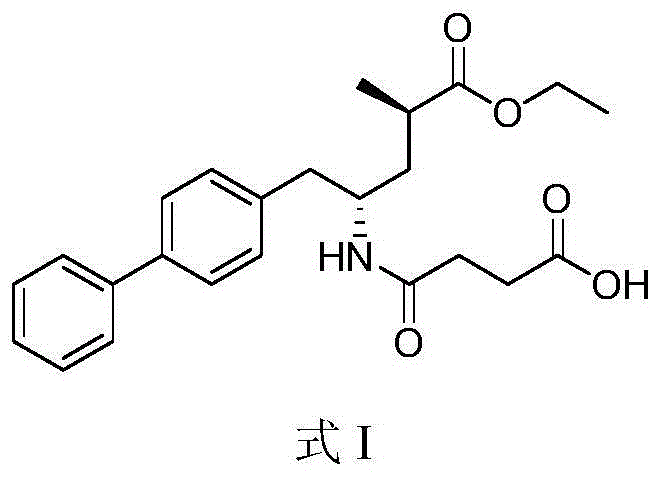
[0084] Dissolve 2.7 g (24.3 mmol) of potassium tert-butoxide in 20 ml of absolute ethanol, and dissolve 10.0 g (24.3 mmol) of sacubi in 100 ml of absolute ethanol. Under stirring, the above-mentioned ethanol solution of potassium tert-butoxide was added dropwise to the ethanol solution of sacubitril. Concentrate under reduced pressure at 40-45°C to a foamy solid. Dissolve the concentrate in 20 ml of isobutanol at 55-60°C, and cool to about 10°C. Filtrate, wash the filter cake with isobutanol, and dry under reduced pressure at 55-60°C to obtain crystalline form B of sacubitril potassium salt.
[0085] 1 H NMR (400MHz, CD 3 OD)δ:1.143-1.161(d,3H),1.206-1.241(t,3H),1.453-1.525(m,1H),1.885-1.966(m,1H),2.414(s,4H),2.564-2.608 (m,1H),2.740-2.817(m,2H),4.059-4.156(m,3H),7.286-7.336(m,3H),7.404-442(t,3H),7.534-7.564(d,2H) ,7.590-7.618(d,2H).
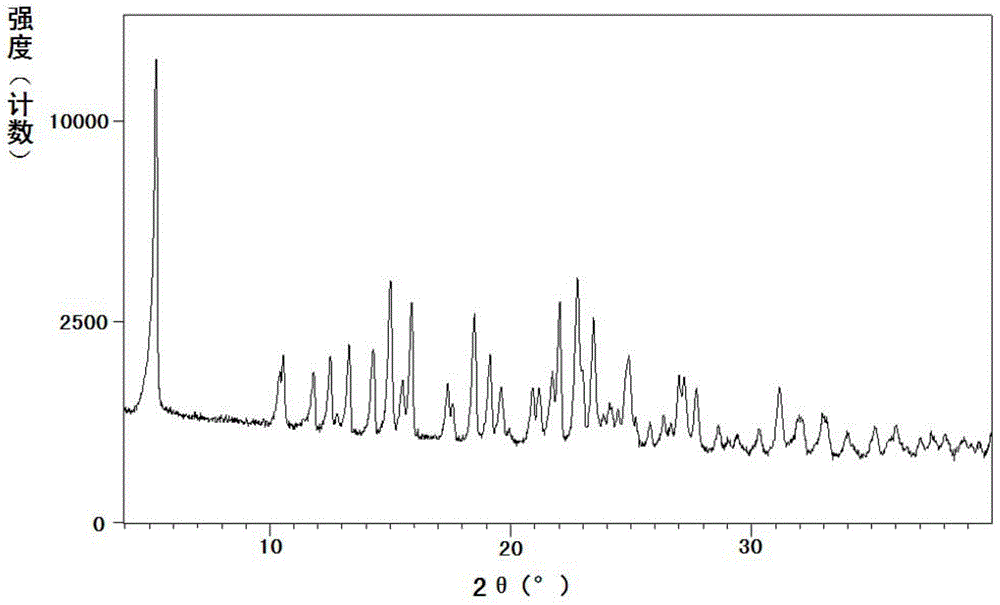
[0086] The measured powder X-ray diffraction pattern is shown in fig...
Embodiment 2
[0090] Example 2: Preparation of Shakubiqu Potassium Salt Form B
[0091] Dissolve 0.12 g (2.19 mmol) of potassium hydroxide in 2 ml of absolute ethanol, and dissolve 1.00 g (2.43 mmol) of sacubi in 10 ml of absolute ethanol. Under stirring, the above-mentioned ethanol solution of potassium hydroxide was added dropwise to the ethanol solution of Shakubiqu. Concentrate under reduced pressure at 40-45°C. Dissolve the concentrate in 5ml of isopropanol at 50-55°C, then add n-heptane dropwise, stop the dropwise addition when a precipitate precipitates and dissolves rapidly again (about 15ml of heptane is consumed), and cool to about -10 ℃. Filtrate, wash the filter cake with n-heptane, and dry under reduced pressure at 40-45°C to obtain the crystal form B of sacubitril potassium salt.
Embodiment 3
[0092] Example 3: Preparation of Shakubiqu Potassium Salt Form B
[0093] Dissolve 0.16 g (1.94 mmol) of potassium ethoxide in 2 ml of absolute ethanol, and 1.00 g (2.43 mmol) of sacubiqu in 10 ml of absolute ethanol. Under stirring, the above-mentioned ethanol solution of potassium ethoxide was added dropwise to the ethanol solution of Sacubitra. Concentrate under reduced pressure at 40-45°C. At 55-60°C, dissolve the concentrate in a mixed solvent composed of 4ml of isobutanol and 10ml of methyl tert-butyl ether, and cool to about 20°C. After filtering, the filter cake was washed with methyl tert-butyl ether to obtain the crystal form B of the potassium salt of Shakubiqu.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com