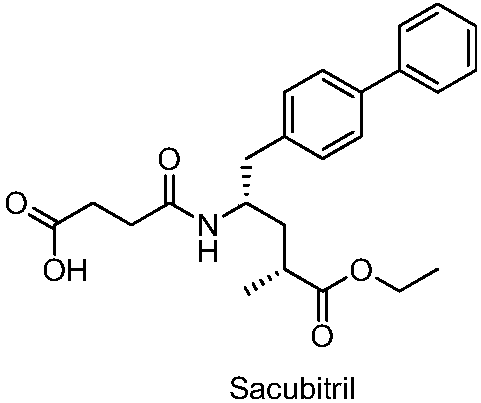
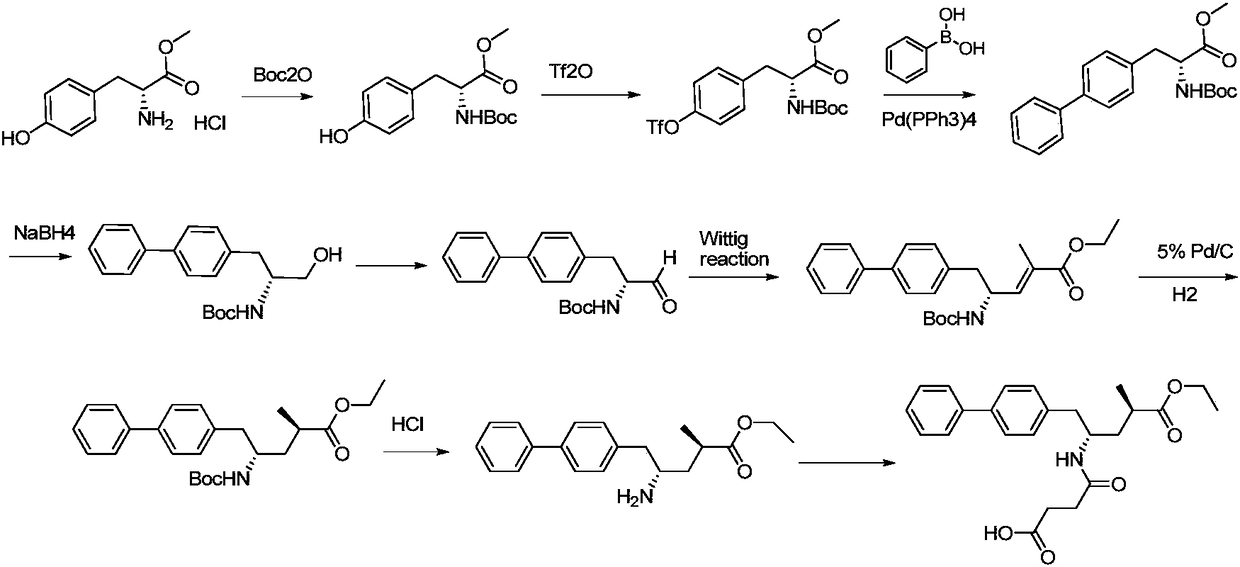
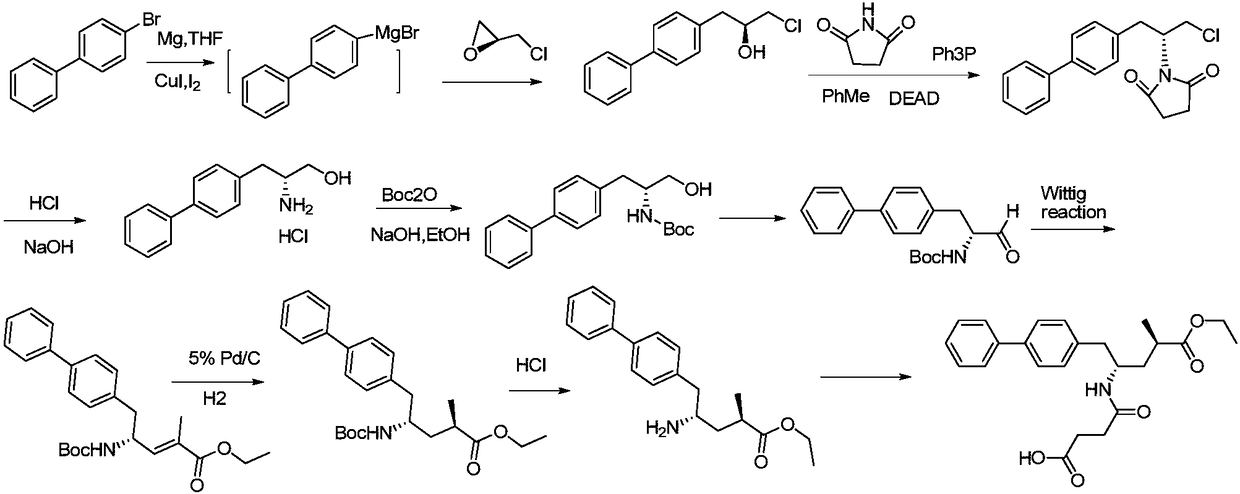
Sacubitril intermediate and preparation method and application thereof
A technology for sacubitril and intermediate, which is applied in the field of new intermediate compound and preparation of sacubitril, can solve the problems such as failure to consider atom economy well, difficult to industrialize, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071]
[0072] Add 50g (0.2mol) (S)-1-([1,1'-biphenyl]-4-yl)-3-chloropropan-2-ol and 600ml toluene solvent to a 1L reaction flask, stir at room temperature, and the system Dissolve, lower the temperature of the reaction system to 0-5°C, add 63.8g of triphenylphosphine (0.24mol, 1.2eq) and 23.76g (0.24mol, 1.2eq) of succinimide, add diisoazodicarboxylate Propyl ester 48.5g (0.3mol, 1.5eq), after the addition is complete, the system is heated to 20-30°C and reacted for 3-5 hours, then 150ml of water is added, stirred at room temperature for 5-10 minutes, and the liquid is separated, and the organic layer is washed with saturated saline (100ml ×2) washed, dried over anhydrous MgSO4, and concentrated to obtain 54.68 g of off-white solid compound IIa with a yield of 82.3%.
Embodiment 2
[0074]
[0075] Add 50g (0.17mol) (S)-1-([1,1'-biphenyl]-4-yl)-3-bromopropan-2-ol and 600ml dichloromethane solvent into a 1L reaction flask, stir at room temperature , the system was dissolved, lowered the temperature of the reaction system to 0-5°C, added 53.5g triphenylphosphine (0.204mol, 1.2eq) and 20.2g (0.204mol, 1.2eq) succinimide, added azodicarboxylic acid 38.4g (0.22mol, 1.3eq) of diethyl ester, after the addition, the temperature of the system was raised to 20-30°C for 3-5 hours, and 150ml of water was added, stirred at room temperature for 5-10 minutes, separated, and the organic layer was washed with saturated saline ( 100ml×2) washed, dried with anhydrous MgSO4, and concentrated to obtain 56.7g of solid compound IIb with a yield of 93.4%.
Embodiment 3
[0077]
[0078] Add 50g (0.15mol) (S)-1-([1,1'-biphenyl]-4-yl)-3-iodopropan-2-ol and 600ml dichloromethane solvent to 1L reaction flask, room temperature Stir to dissolve the system, lower the temperature of the reaction system to 0-5°C, add 47.2g of triphenylphosphine (0.18mol, 1.2eq) and 17.8g (0.18mol, 1.2eq) of succinimide, add azobis Diethyl formate 33.9g (0.195mol, 1.3eq), the addition is complete, the system is warmed up to 20-30°C and reacted for 3-5 hours, add 150ml of water, stir at room temperature for 5-10 minutes, separate the liquid, and the organic layer is washed with saturated saline (100ml×2) washed, dried over anhydrous MgSO4, and concentrated to obtain 56.1g of solid compound IIc with a yield of 90.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com