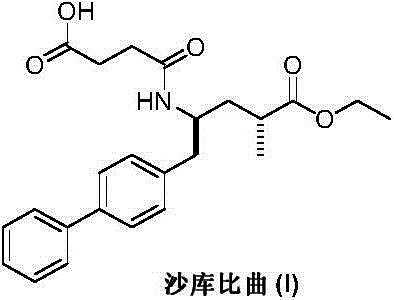
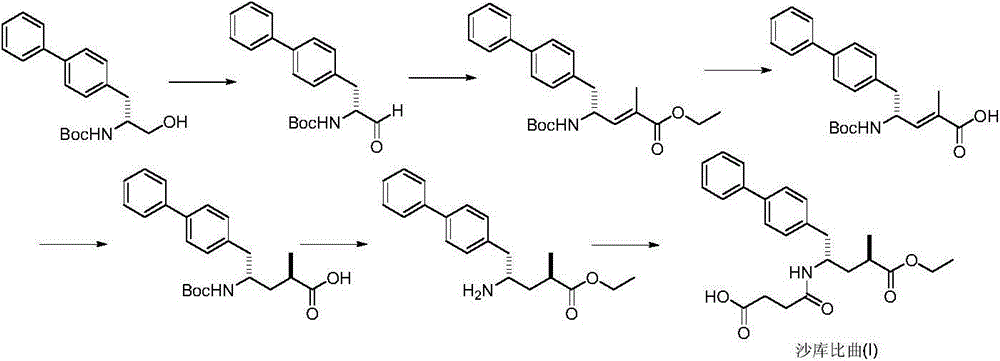
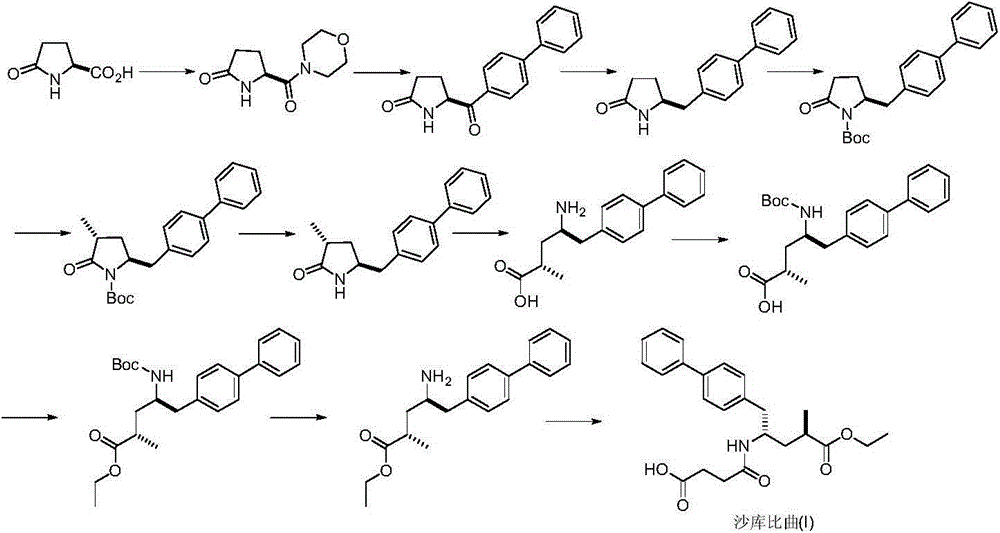
Preparation method of sacubitril
A technique of sacubiqu and preparation steps, which is applied in the field of preparation of neprilysin inhibitor sacubiqu, can solve problems such as difficult industrialization, difficult industrialization, and numerous reaction steps, and achieves environmentally friendly, economical and simple process , the effect of promoting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under nitrogen atmosphere at 0-5°C, (4S,1'R)-4,5-dihydro-2-(1-methyl-3-butenyl)-4-(1-methylethyl) Oxazole (16.7g, 0.1mol) was added in hydrochloric acid (10%w / w, 250mL), heated to boiling water, stirred for 3 hours, cooled to room temperature, extracted three times with dichloromethane, recovered solvent under normal pressure, and reduced Pressure distillation afforded 2R-methyl-4-ene-pentanoic acid as a yellow liquid. The obtained yellow oil was dissolved in 250 mL of dichloromethane, cooled to -78°C, and ozone was introduced, the color of the solution gradually changed to dark green, and the reaction ended after about 15 minutes. Use nitrogen to remove excess ozone, raise to room temperature, wash with 5% sodium thiosulfate aqueous solution and pure water successively, dry over anhydrous sodium sulfate, recover solvent to obtain light yellow oil 2R-methyl-4-oxo-butanol Acid (III) 7.9g, yield 68.1%; FAB-MSm / z: 117[M+H] + .
Embodiment 2
[0027] Add (S)-1-(α-aminobenzyl)-2-naphthol (II) (7.5g, 30mmol), 2R-methyl-4-oxo-butanoic acid (III) (3.7 g, 31.5mmol) and toluene 100mL, heated up to 40-50°C, stirred and reacted for 36 hours, and TLC detected that the reaction was complete. Cool down and remove insoluble matter by filtration. Concentrate to recover toluene, and the resulting oil is recrystallized with isopropyl ether and dried in vacuo to obtain a white solid (7aR, 9R, 12S)-9-methyl-10-oxo-12-phenyl-7a,8,9,10- Tetrahydro-12H-naphtho[1,2-e]pyrrolecyclo[2,1-b][1,3]oxazine (IV) 7.2g, yield 72.9%; 1H NMR (CDCl 3)δ7.72-765 (m, 2H), 7.35-7.28 (m, 1H), 7.24-7.13 (m, 7H), 7.10 (d, 1H), 5.84 (s, 1H), 5.65-5.54 (m, 1H), 2, 43-2.67(m, 1H), 2.24-2.57(m, 2H), 1.16(d, 3H); FAB-MSm / z: 330[M+H] + .
Embodiment 3
[0029] At room temperature and under a nitrogen atmosphere, add a small amount of bromoethane (initiator), magnesium powder (1.0 g, 40 mmol) and 10 mL of dry tetrahydrofuran into a dry reaction flask. After the reaction is initiated, add 4-bromomethyl-1,1 '-biphenyl (4.92g, 20mmol) in 100mL of dry tetrahydrofuran solution, after the dropwise addition, continue to stir for 3-4 hours to prepare the Grignard reagent (1,1'-biphenyl-4-yl-methyl) bromide magnesium oxide. Cool down to -78°C, add dropwise (7aR,9R,12S)-9-methyl-10-oxo-12-phenyl-7a,8,9,10-tetrahydro-12H-naphtho[1,2 -e] A solution of pyrrocyclo[2,1-b][1,3]oxazine (IV) (4.9g, 15mmol) in 100mL of dry tetrahydrofuran was maintained at the temperature for 2-3 hours, and the reaction was detected by TLC. The reaction was quenched with 50 mL of saturated ammonium chloride, raised to room temperature, extracted three times with dichloromethane, and the organic phases were combined and dried over anhydrous magnesium sulfate. C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com