Sacubitril derivatives and medicine compositions, preparation methods and application thereof
A technology of sacubitril and sacubitril, applied in pharmaceutical composition, preparation of drugs for preventing or treating chronic heart failure and/or hypertension, sacubitril derivatives, its crystal form, preparation field, It can solve the problems of unstable crystal form and long preparation period of crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
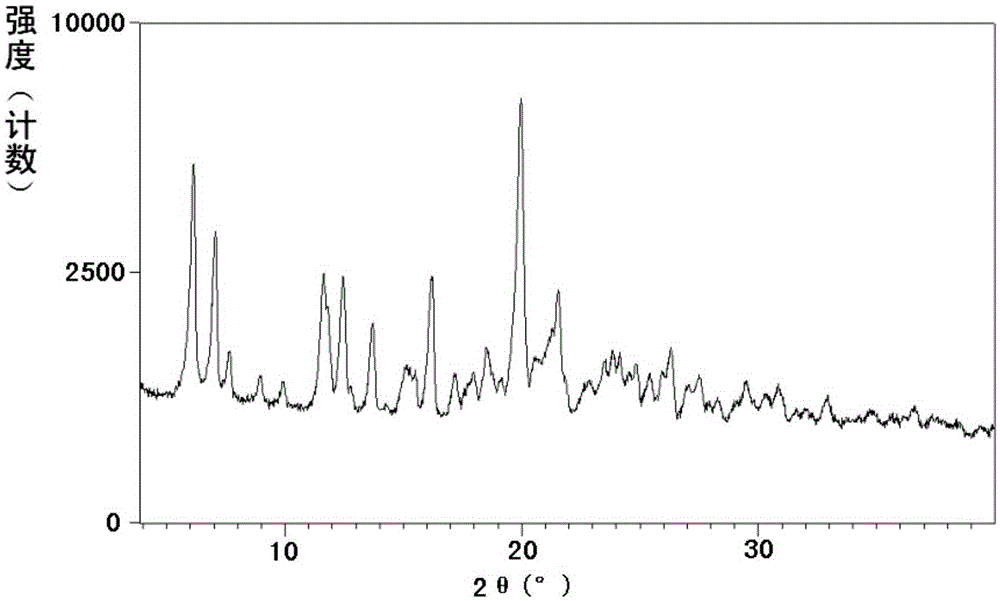
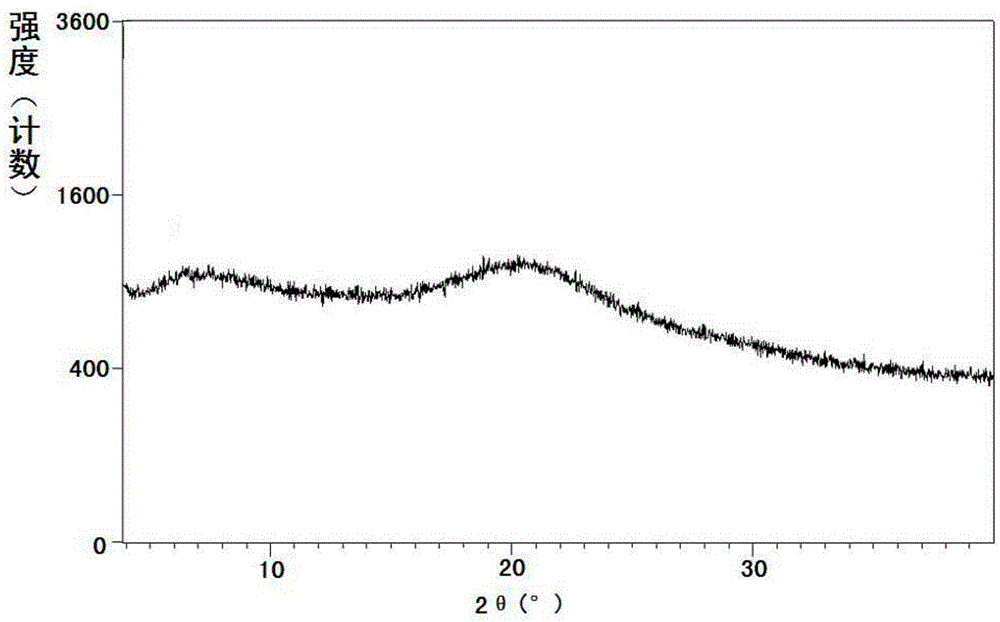
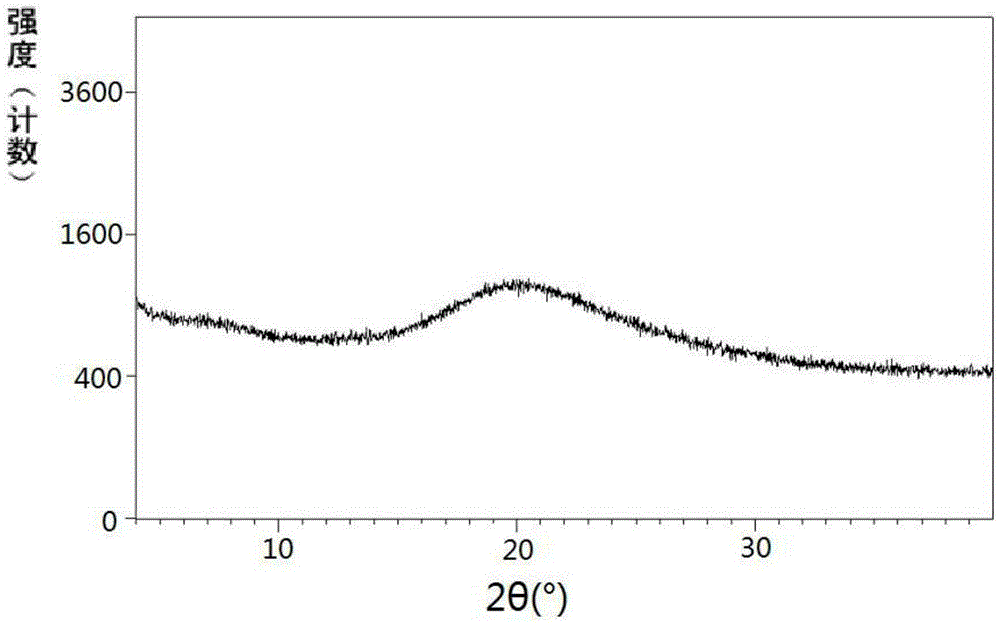
[0364] Example 1: Preparation of Shakubiqu potassium salt and its crystal form A
[0365] Dissolve 1.4 g (24.3 mmol) of potassium hydroxide in 20 ml of absolute ethanol, and dissolve 10.0 g (24.3 mmol) of sacubi in 100 ml of absolute ethanol. Under stirring, the above-mentioned ethanol solution of potassium hydroxide was added dropwise to the ethanol solution of Shakubiqu. Concentrate under reduced pressure at 40-45°C to a foamy solid. Dissolve the concentrate in 100ml of toluene at 55-60°C, then add n-heptane dropwise, stop the dropwise addition when a precipitate precipitates and dissolves rapidly again (about 100ml of n-heptane is consumed), and cool to about 10°C. Filtrate, wash the filter cake with methyl tert-butyl ether, and dry under reduced pressure at 55-60°C to obtain the potassium salt of sacubitril.
[0366] 1 HNMR (400MHz, DMSO-d 6 ( m,2H),3.891-4.017(m,3H),7.250-7.271(d,2H),7.319-7.355(m,1H),7.428-7.466(m,2H),7.561-7.581(d,2H), 7.639-7.660(d,2H).
[0367]...
Embodiment 2
[0371] Example 2: Preparation of Shakubiqu potassium salt and its crystal form A
[0372] Dissolve 0.12 g (2.19 mmol) of potassium hydroxide in 2 ml of absolute ethanol, and dissolve 1.00 g (2.43 mmol) of sacubi in 10 ml of absolute ethanol. Under stirring, the above-mentioned ethanol solution of potassium hydroxide was added dropwise to the ethanol solution of Shakubiqu. Concentrate under reduced pressure at 40-45°C. Dissolve the concentrate in 5ml of acetone at 50-55°C, then add methyl tert-butyl ether dropwise, stop the dropwise addition when a precipitate precipitates out and dissolves rapidly again (about 20ml of methyl tert-butyl ether is consumed), Cool to about -10°C. After filtering, the filter cake was washed with methyl tert-butyl ether, and dried under reduced pressure at 40-45°C to obtain the crystal form A of the potassium salt of Shakubiqu.
Embodiment 3
[0373] Example 3: Preparation of Shakubiqu potassium salt and its crystal form A
[0374] Dissolve 0.16 g (1.94 mmol) of potassium ethoxide in 2 ml of absolute ethanol, and 1.00 g (2.43 mmol) of sacubiqu in 10 ml of absolute ethanol. Under stirring, the above-mentioned ethanol solution of potassium ethoxide was added dropwise to the ethanol solution of Sacubitra. Concentrate under reduced pressure at 40-45°C. At 55-60°C, dissolve the concentrate in a mixed solvent composed of 20ml of ethyl acetate and 35ml of methyl tert-butyl ether, and cool to about 20°C. After filtering, the filter cake was washed with methyl tert-butyl ether to obtain the crystalline form A of the potassium salt of Sacubitril.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com