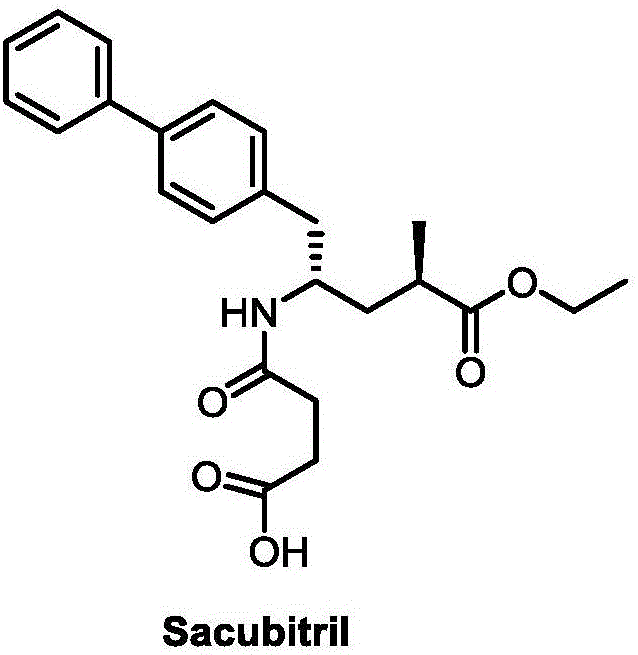
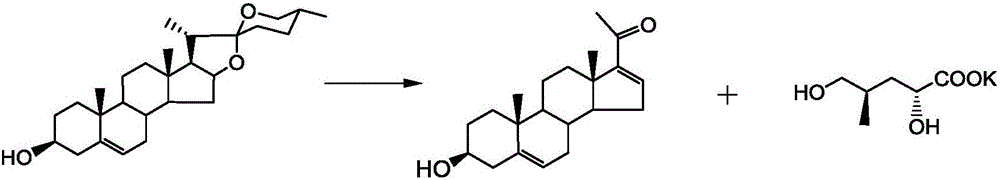
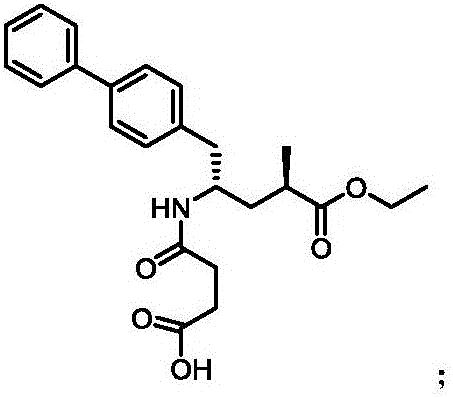
Synthetic method for Sacubitril
A synthetic method, the technology of Sacubitril, applied in the field of Kubitril, can solve the difficulties in the source of chiral auxiliary reagents Bety base and 2R-methyl-4-oxobutanoic acid, the high price of chiral raw materials Eliminate the problems of expensive reagents, etc., and achieve the effect of cheap and easy to obtain, convenient and easy to obtain raw materials, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of Example 1 Compound 3
[0029]
[0030] Dissolve 10g of compound 2 (40mmol) in 100mL of dichloromethane, add 14mL of DBU (2.4eq) and 6mL of RfSO at 0°C 2 F (1.2eq), stirred at 0°C for 3h, quenched with water, extracted the aqueous phase with dichloromethane three times, combined the organic phases and washed with saturated brine once, dried over anhydrous sodium sulfate and filtered, evaporated the solvent under reduced pressure , and separated by column chromatography to obtain compound 3 (8.2 g, yield 88%).
[0031] [α] D 23 +5.3 (c 0.82, CHCl 3 );
[0032] IR (cm -1 ):3410(OH);
[0033] 1H NMR (400MHz, CDCl 3 )3.42-3.52(m,2H),2.94-2.99(m,1H),2.77(t,J=4.4Hz,1H),2.46(dd,J=5.1,2.7Hz,1H),1.82-1.90(m ,1H),1.64-1.70(m,1H),1.27-1.33(m,1H),0.97(d,J=6.7Hz,3H),0.89(s,9H),0.04(s,6H);
[0034] 13 C NMR (100MHz, CDCl 3 )δ68.3,51.2,47.7,36.5,34.1,26.1,18.5,16.8,-5.2,-5.3; HRMS-EI(m / z):[M-C(CH 3 ) 3 ] + calcd for C 8 h 17 o 2 Si:173.0998,found:173.09...
Embodiment 2
[0035] Synthesis of Example 2 Compound 3
[0036]
[0037]Dissolve 10g of compound 2 (40mmol) in 100mL of dichloromethane, add 13mL of pyridine (4eq) and 15g of p-toluenesulfonyl chloride (2eq) at room temperature, stir at room temperature for 3h, add water to quench, dichloromethane to extract the aqueous phase The organic phases were combined three times and washed once with saturated brine, dried over anhydrous sodium sulfate and filtered. The solvent was evaporated under reduced pressure and separated by column chromatography to obtain compound 3 (8.4 g, yield 90%).
Embodiment 3
[0038] Synthesis of Example 3 Compound 3
[0039]
[0040] Dissolve 10 g of compound 2 (40 mmol) in 100 mL of chloroform, add 33 mL of triethylamine (6 eq) and 9 mL of methanesulfonyl chloride (3 eq) at room temperature, stir at room temperature for 4 h, add water to quench, dichloromethane extracts water After three phases, the organic phases were combined and washed once with saturated brine, dried over anhydrous sodium sulfate and filtered. The solvent was evaporated under reduced pressure and separated by column chromatography to obtain compound 3 (8.1 g, yield 87%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com