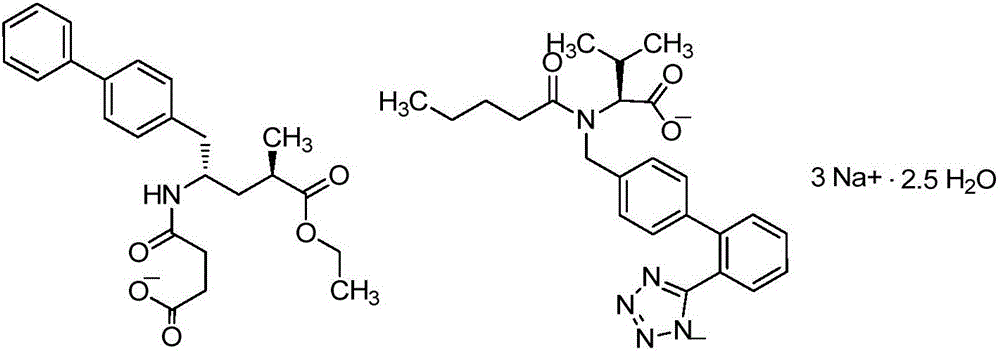
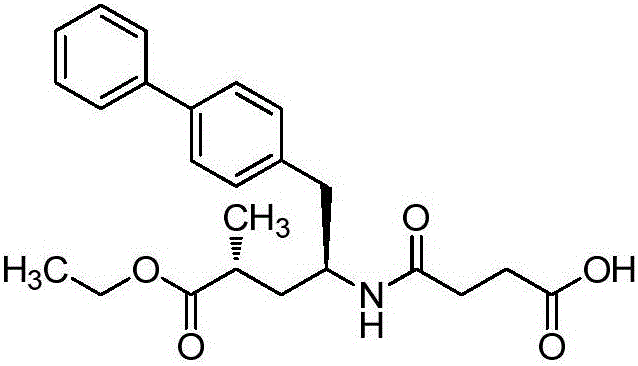
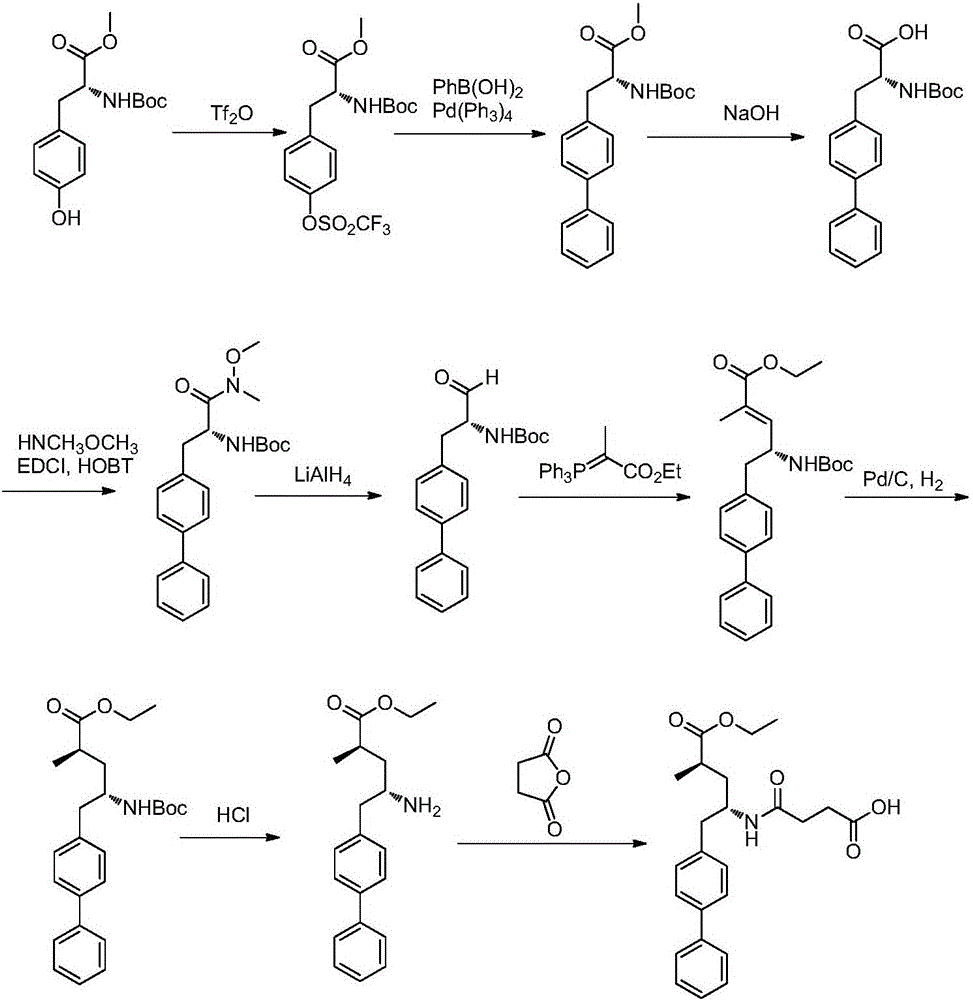
Novel synthesis method of key component Sacubitril of novel anti-heart-failure drug
A technology of sakubiqu and key components, applied in the field of medicine and chemical industry, can solve the problems of high process amplification cost, high process cost, low selectivity, etc., and achieves reduced process cost, low process experimental condition requirements, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Add triphenylphosphine (28.85g, 110mmol) and dichloromethane (129mL) into the three-necked flask, stir to dissolve, cool to 0-5°C, add bromine (17.58g, 110mmol) dropwise, raise to room temperature and stir for 30 Minutes later, compound formula 1 (12.92 g, 100 mmol) was added and reacted at room temperature for 10-16 hours. At the end of the reaction, sodium bisulfite solution (5%, 129 mL) was added to quench the reaction, stirred and separated, the aqueous phase was extracted twice with dichloromethane (65 mL), and the combined organic phase was washed once with saturated brine (65 mL). After drying over sodium sulfate and concentrating, compound 2a (16.52 g, 86%) was obtained by column chromatography with a mixed solvent of petroleum ether and ethyl acetate. ESI m / z=192.0,194.1(M+1) 1 H NMR (400MHz, CDCl 3 )4.05-3.80(m,1H),3.55-3.20(m,2H),2.68-2.43(m,1H),2.15-2.00(m,1H),1.65-1.54(m,1H),1.18(d, J=6.8Hz,3H);
[0041] In embodiment 1, replace triphenylpho...
Embodiment 2
[0043]
[0044] Add compound formula 1 (28.85g, 110mmol) and dichloromethane (140mL) into the three-necked flask, stir and dissolve, add triethylamine (18.18g, 150mmol), stir for 5 minutes, then drop into the dichloromethane solution of p-toluenesulfonyl chloride (20.97g, 110mmol, dissolved in dichloromethane), react at room temperature for 8-10 hours. After the reaction was completed, water (140 mL) was added to quench the reaction, stirred and separated, the aqueous phase was extracted twice with dichloromethane (140 mL), the combined organic phase was washed once with saturated brine (70 mL), dried over anhydrous sodium sulfate, concentrated and used The compound 2b (24.93 g, 88%) was separated by column chromatography with a mixed solvent of petroleum ether and ethyl acetate. ESI m / z=284.2(M+1) 1 H NMR (400MHz, CDCl 3)δ7.80(d, J=8.4Hz, 2H), 7.39(d, J=8.0Hz, 2H), 4.10-4.00(m, 1H), 3.95-3.75(m, 2H), 2.70-2.45(m ,1H),2.45(s,3H),2.05-1.90(m,1H),1.60-1.40(m,1H),1.16(d,J=6...
Embodiment 3
[0047]
[0048] Under nitrogen protection, add 4-bromobiphenyl (6.99g, 30mmol), magnesium chips (5.34g, 220mmol), 1-2 grains of iodine and tetrahydrofuran (70mL) into the three-neck flask A, stir well and cool to 0-5°C , switch 3 times with nitrogen, heat to reflux until the color of iodine disappears, Grignard reaction is triggered, slowly continue to drop 4-bromobiphenyl tetrahydrofuran solution (41.96g, 180mmol, dissolved in 80mLTHF and degassed) to keep the solution slightly boiling, add After completion, it was raised to 55-60°C for 1-2 hours, and after the reaction, it was cooled to 0-5°C for later use; in another three-necked flask B, compound 2a (19.21g, 100mmol), catalyst iron triacetylacetonate (0.96g) and Tetrahydrofuran (40mL), stir and dissolve, cool to 0-5°C and switch nitrogen for 3 times under vacuum, transfer the biphenylmagnesium bromide solution in bottle A into reaction bottle B with a syringe, and warm up to room temperature for reaction after dropping ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com