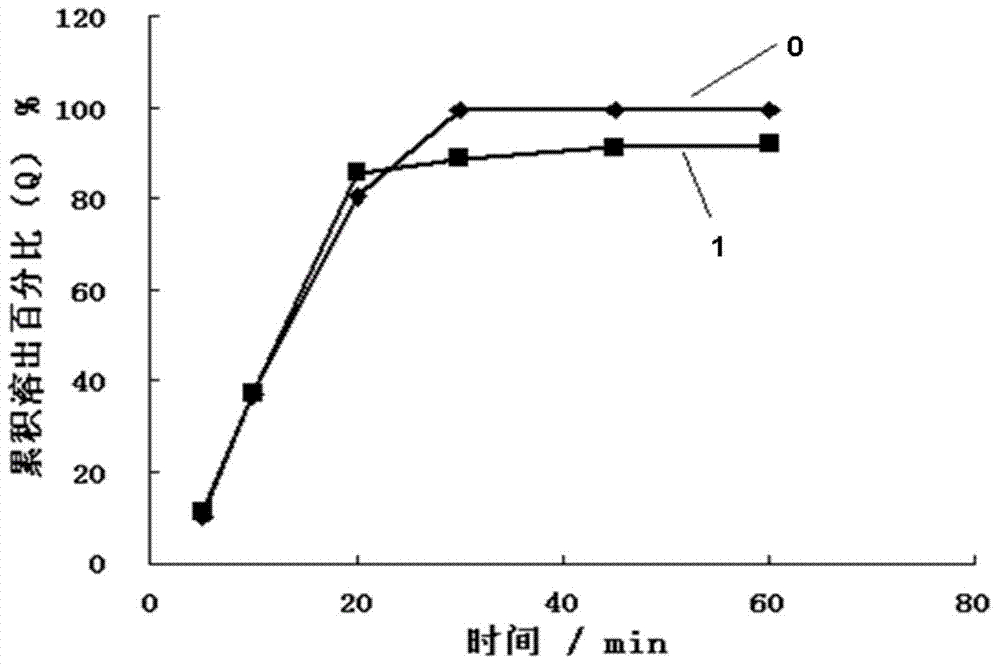
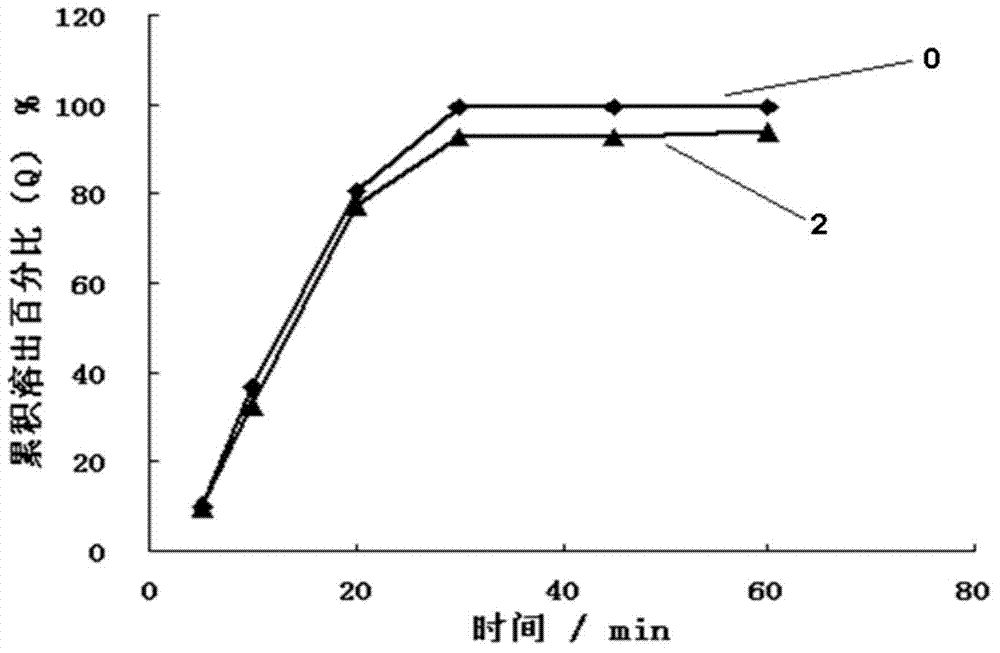
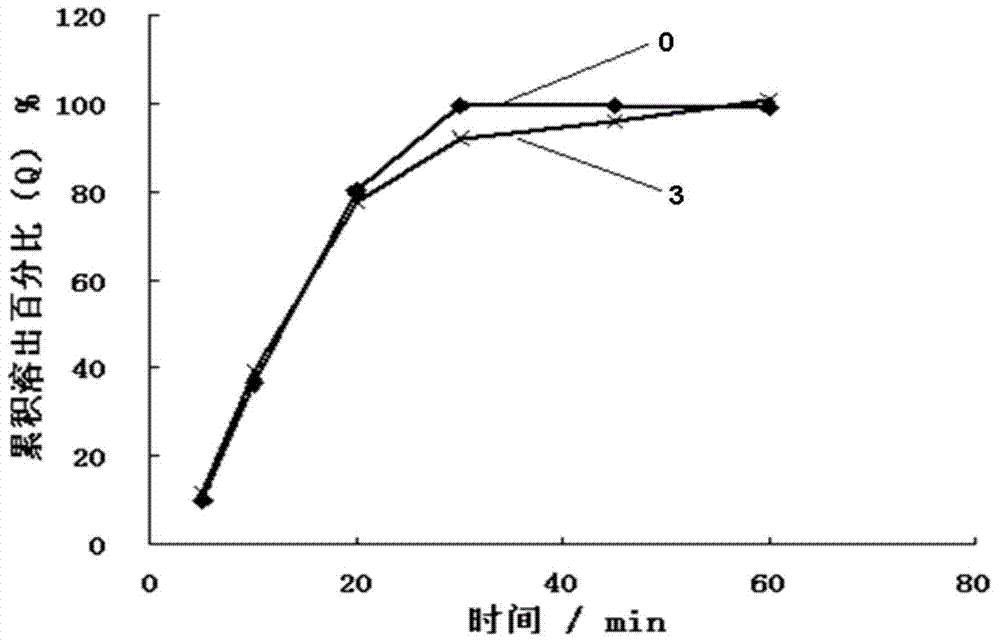
Nimodipine solid dispersant and tablet and their preparation methods
A nimodipine solid and dispersant technology, applied in the field of medicine, can solve the problems of uneven particle size, complicated process, small particle size, etc., and achieve the effects of high bioavailability, simple preparation steps, and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] (1) Add 300g of microcrystalline cellulose (MCC), 100g of starch and 50g of crospovidone (PVPP) into the fluidized bed as a filler;
[0066] (2) Add 30g of nimodipine and 30g of povidone K25 (PVP K25) into 800g of ethanol and dissolve completely;
[0067] (3) Spray the above solution evenly on the filler in the fluidized bed, the atomization pressure of the fluidized bed is 1.8bar, the spraying speed of the fluidized bed is 6g / min, and the air intake volume during granulation is 30m 3 / h, the inlet air temperature is 60°C during granulation, and the drying time is 30 minutes after granulation, and the air inlet volume during drying is 40m 3 / h, the air inlet temperature is 70°C, the particles are dried and discharged to obtain the composition;
[0068] (4) Add 8 g of micropowder silica gel, 20 g of croscarmellose sodium and 4 g of magnesium stearate to 660 g of the prepared composition, mix well, press into tablets, and coat.
[0069] The marked amount per tablet is 3...
Embodiment 2
[0071] (1) Add 50g of microcrystalline cellulose (MCC) and 10g of starch into the fluidized bed as filler;
[0072] (2) Add 30g of nimodipine and 180g of povidone K30 (PVP K30) into a mixed solvent composed of 300g of chloroform and 10g of methanol to dissolve completely;
[0073] (3) Spray the above solution evenly on the filler in the fluidized bed, the atomization pressure of the fluidized bed is 1.5bar, the spraying speed of the fluidized bed is 5g / min, and the air intake volume during granulation is 25m 3 / h, the air inlet temperature is 40°C during granulation, and it is dried for 40 minutes after granulation, and the air inlet volume during drying is 30m 3 / h, the air inlet temperature is 65°C, the particles are dried and discharged, and the composition is obtained;
[0074] (4) Add 10 g of talc powder, 35 g of crospovidone (PVPP) and 5 g of stearic acid to 270 g of the prepared composition, mix well, press into tablets, and coat.
[0075] The marked amount per tablet...
Embodiment 3
[0077] (1) Add 100g of microcrystalline cellulose (MCC), 50g of lactose and 60g of mannitol into the fluidized bed as filler;
[0078] (2) Add 30g of nimodipine and 105g of povidone K25 (PVP K25) into a mixed solvent composed of 250g of ethyl acetate and 50g of acetone to completely dissolve;
[0079] (3) Spray the above solution evenly onto the filler in the fluidized bed, the atomization pressure of the fluidized bed is 1.5bar, the spraying speed of the fluidized bed is 7g / min, and the air intake volume during granulation is 35m 3 / h, the inlet air temperature is 30°C during granulation, and the drying time is 30 minutes after granulation, and the air inlet volume during drying is 35m 3 / h, the air inlet temperature is 40°C, the granules are dried in the fluidized bed, and then transferred to an oven for 5 hours to continue drying, the drying temperature is 50°C, and the material is discharged after drying to obtain the composition;
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com