Nimoldipine new nano liposome, its precursor freeze dryed matter and its preparing method
A technology of nimodipine nanometer and nimodipine, which is applied in freeze-drying delivery, drug combination, pharmaceutical formulation and other directions, can solve the problems of large particle size of liposomes and poor stability of lipid nanoparticles, and achieve high quality stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
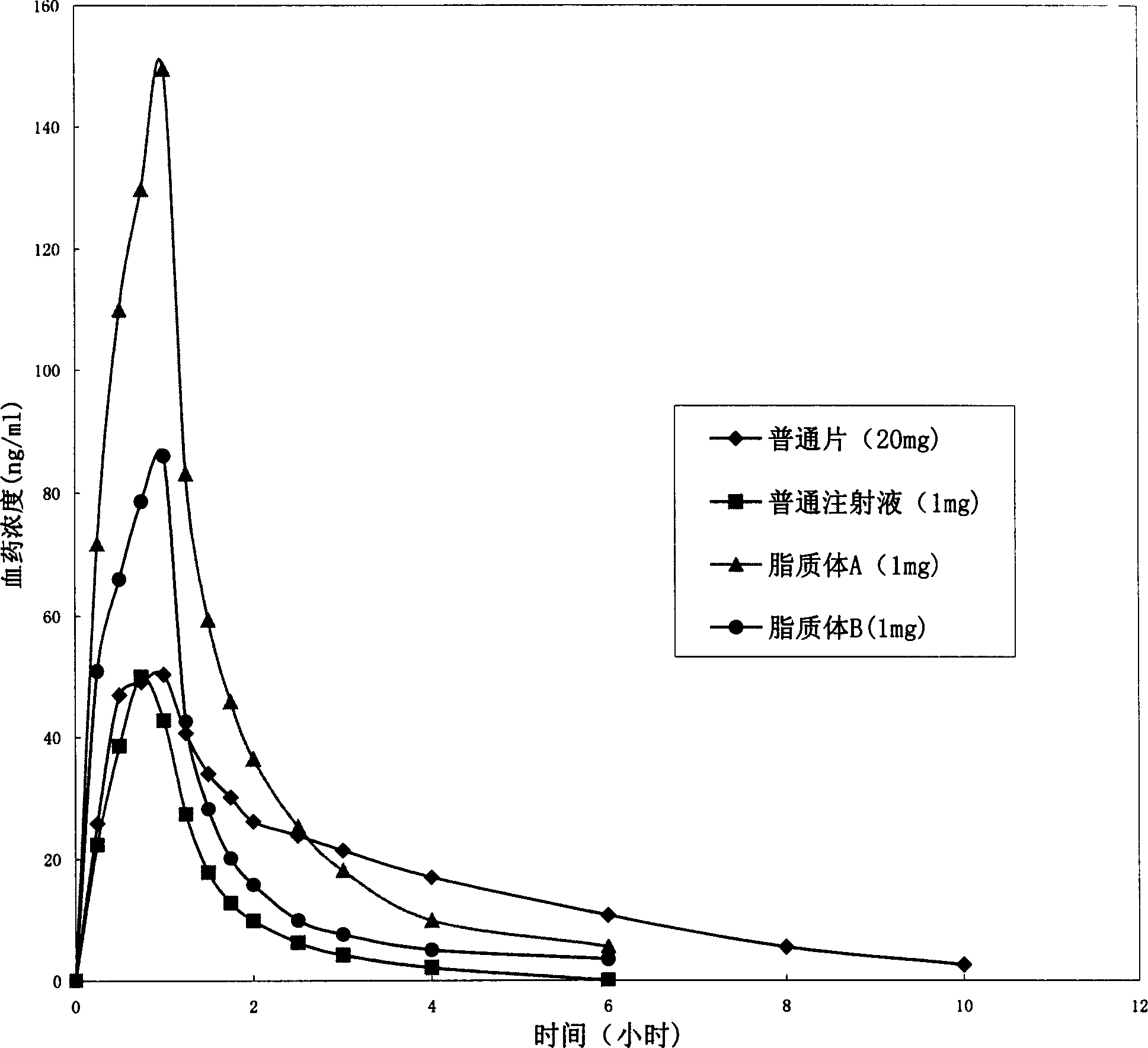
Image
Examples
Embodiment 1
[0083] Preparation of Nimodipine Nanoliposomes:
[0084] Nimodipine 10mg
[0085] Hydrogenated egg yolk phospholipids 150mg
[0086] Cholesterol 20mg
[0087] poloxamer188 200mg
[0088] Sodium deoxycholate 10mg
[0089] Weigh the prescribed amount of nimodipine, hydrogenated egg yolk phospholipids, cholesterol, poloxamer188, sodium deoxycholate, etc., and dissolve them in 3ml of absolute ethanol to obtain a lipid solution, transfer it to a 1000ml eggplant-shaped bottle, and keep the temperature at 60°C In a water bath, use a rotary thin film evaporator to remove ethanol and form a uniform lipid film on the bottle wall. Measure 10ml of water and medium, pour it into an eggplant-shaped bottle, shake it gently, keep a constant temperature of 60°C, elute the lipid film and disperse it in the hydration medium to dissolve, and then obtain a liposome suspension, put it in a water bath for ultrasound Ultrasound to a translucent colloidal solution (keep in a 60°C water bath), and...
Embodiment 2
[0091] Preparation of Nimodipine Novel Nano-Liposome Precursor Lyophilizate
[0092] Prescription is the same as embodiment 1
[0093] Preparation process: After obtaining the liposome suspension with Example 1, place it in a refrigerator at -20°C for 12 hours, then freeze-dry to remove the solvent, and obtain the lyophilized product of nimodipine nano-liposome precursor. Nitrogen filled and sealed, protected from light.
Embodiment 3
[0095] Preparation of Nimodipine Nanoliposome Precursor Lyophilizate
[0096] prescription:
[0097] Nimodipine 10mg
[0098] Egg yolk phospholipids 250mg
[0099] Cholesterol 30mg
[0100] poloxamer188 100mg
[0101] Polyoxyethylene castor oil 50mg
[0102] Sodium deoxycholate 15mg
[0103] Mannitol 500mg
[0104] Leucine 150mg
[0105] Preparation process: with embodiment 1,2. The encapsulation efficiency of the obtained nimodipine nano liposome is 89.1%, and the average particle diameter is 20.3nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com