Nimodipine pulse sustained release mircopill preparation and preparing method thereof
A technology of sustained-release pellets and pulses, which is used in pharmaceutical formulations, medical preparations containing active ingredients, cardiovascular system diseases, etc. problems, to achieve the effect of reducing the frequency of medication, reducing side effects, and facilitating medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1. nimodipine contains pill core, and composition is as follows:
[0047] Nimodipine 24g
[0048] Lactose 41g
[0049] Microcrystalline Cellulose 20g
[0050] Bolosham 4g
[0051] water 30g
[0052] Preparation:
[0053] Mix nimodipine, lactose, and microcrystalline cellulose evenly, make a soft material with boloxamer aqueous solution, place it on an extrusion spheronizer, extrude, and the extrusion speed is 14Hz; spheronize, the spheronization speed is 23Hz 0.5min , 17Hz 2.5min; drying at 60°C.
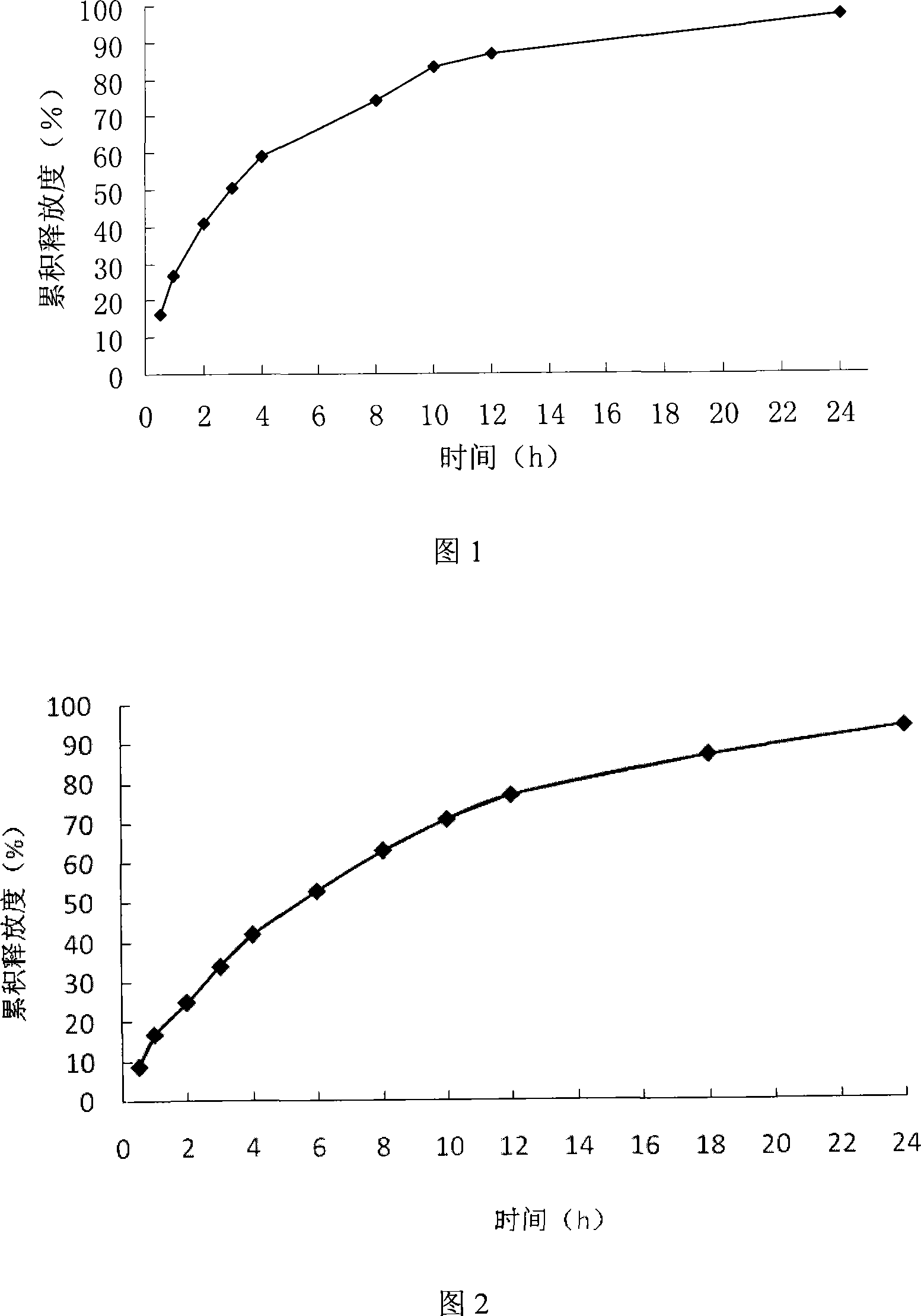
[0054] In vitro release test
[0055] Adopt the device of " Chinese Pharmacopoeia 2005 edition two appendices XC dissolution test method first method, get nimodipine sustained-release pellets (equivalent to nimodipine 120mg) six parts, be 1.0 with 1000mL containing 30% isopropanol pH The hydrochloric acid solution is the release medium, the rotation speed is 100 revolutions per minute, 5mL samples are taken at a certain time, and the same temperature and the...
Embodiment 2
[0057] Example 2. Sustained-release pellets with stomach-soluble coating layer
[0058] Composition of pill core: (based on 200 capsules)
[0059] Nimodipine 24g
[0060] Sodium Lauryl Sulfate 4g
[0061] Lactose 16g
[0062] Microcrystalline Cellulose 45g
[0063] water 40g
[0064] Coating material:
[0065] Eudragit E PO 9g
[0066] Preparation:
[0067] Mix nimodipine, sodium lauryl sulfate, lactose, and microcrystalline cellulose evenly, make a soft material with an aqueous solution, place it on an extrusion spheronizer, extrude, spheronize, dry at 60°C, and use Eudragit E PO Aqueous dispersion for coating. Sustained-release pellets with a gastric-soluble coating layer were obtained.
[0068] Fill the above-mentioned coated sustained-release pellets into empty capsules, each containing 120 mg of nimodipine, to obtain a capsule of sustained-release pellets.
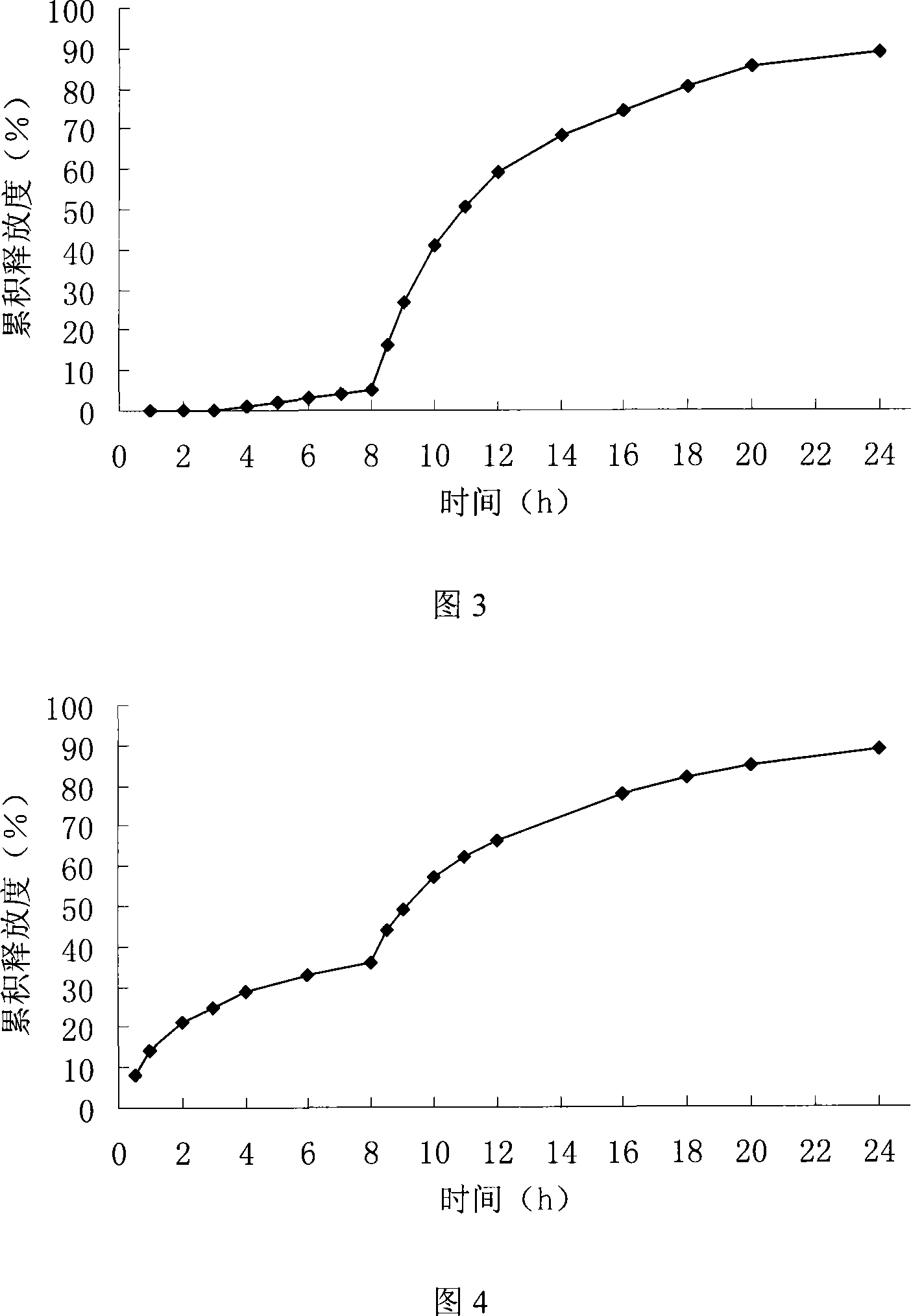
[0069] In vitro release test
[0070] Get 6 capsules, test according to the method under the in vitro re...
Embodiment 3
[0072] Example 3. Sustained-release pellets with enteric coating layer
[0073] Composition of pill core: (based on 200 capsules)
[0074] Nimodipine 24g
[0075] Sodium Lauryl Sulfate 6g
[0076] Lactose 37g
[0077] Microcrystalline Cellulose 29g
[0078] water 35g
[0079] Coating material:
[0080] Eudragit S100 20g
[0081] Preparation:
[0082] Mix nimodipine, sodium lauryl sulfate, lactose, and microcrystalline cellulose evenly, make a soft material with an aqueous solution, place it on an extrusion spheronizer, extrude, and spheronize; dry; use Eudragit S100 water dispersion Perform coating. Sustained-release pellets coated with enteric coating layer were obtained.
[0083] The above-mentioned coated sustained-release pellets are filled in empty capsules, each containing 120 mg of nimodipine. Capsules of sustained-release pellets.
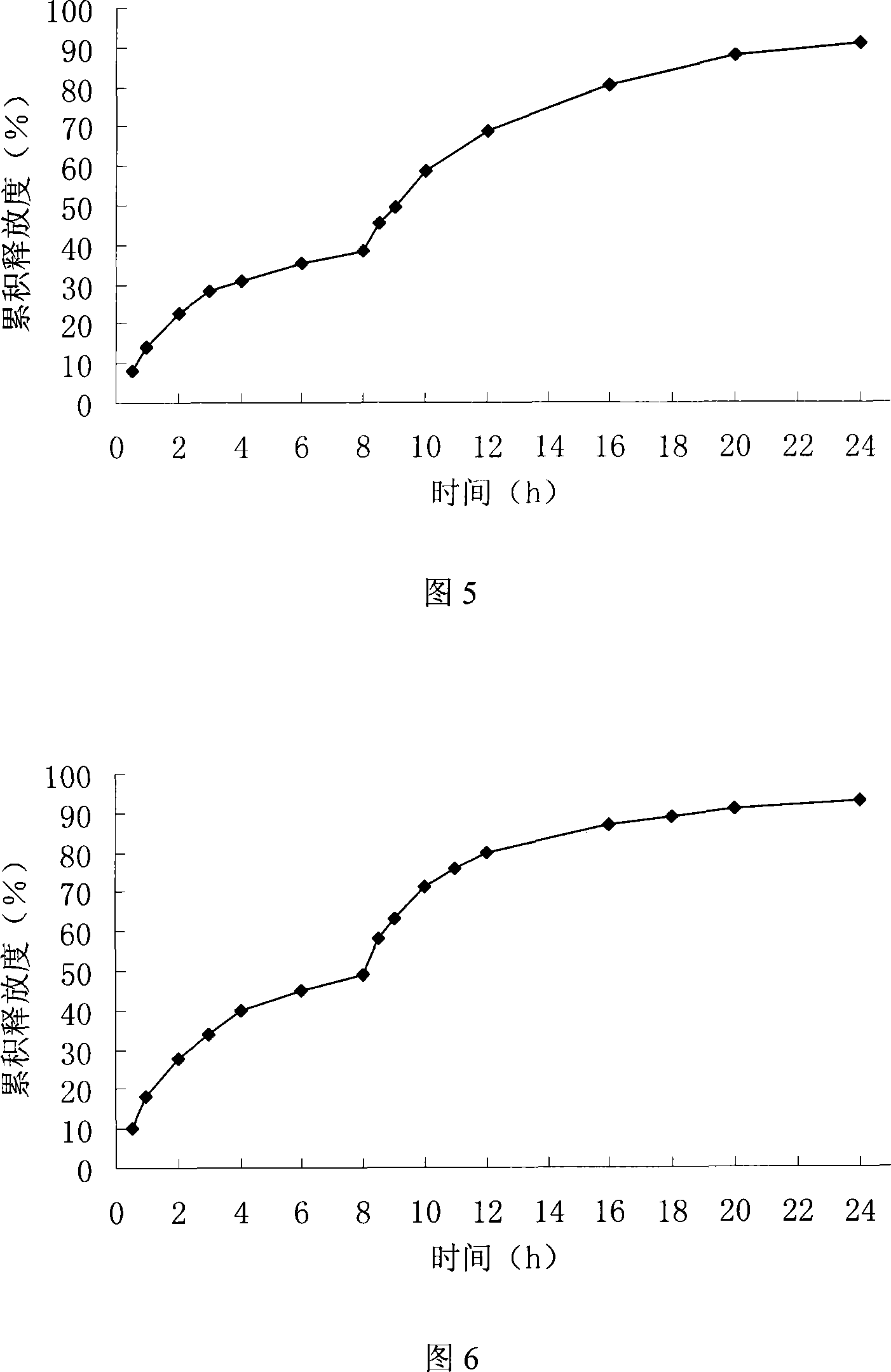
[0084] In vitro release test
[0085] The test was carried out according to the method under the in vitro release test item in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com