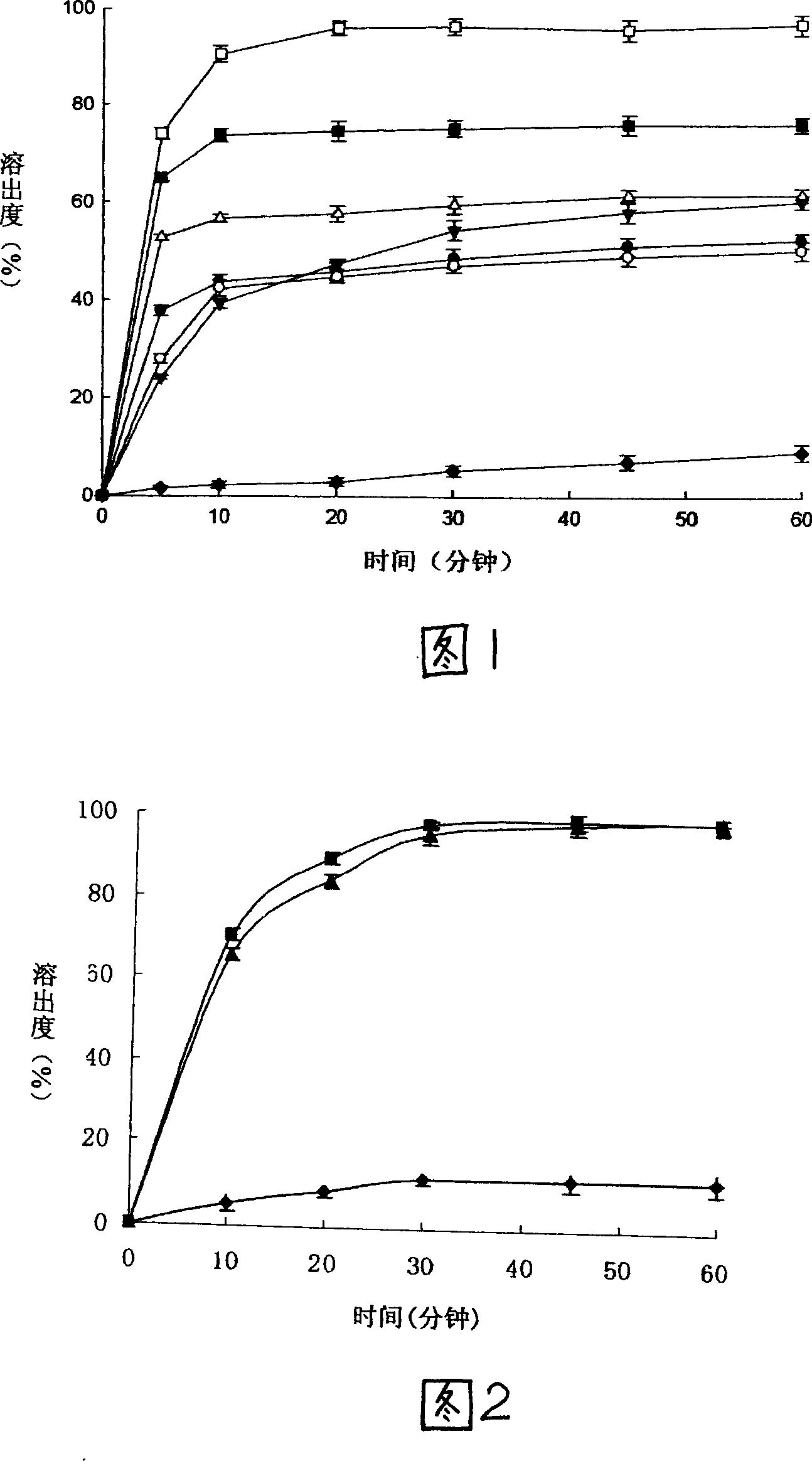
Method for preparing nimodipine dispersible tablet with high dissolution
A technology of nimodipine and nimodipine, applied in the field of preparation of high-dissolution nimodipine dispersible tablets, can solve the problems of decreased bioavailability, complicated preparation process, low inclusion rate, etc., and achieves the improvement of oral biological Effects of availability, avoidance of drug metabolic inactivation, and ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Preparation of nimodipine solid solution: take copovidone S630 as the carrier, premix with nimodipine according to the ratio of 9:1 and 5 minutes, and the temperature of the melt extruder from the first section to the machine head is respectively Set to 90°C-130°C-130°C-130°C-130°C (specifically, the temperature of the first section is set to 90°C, the second section is set to 130°C, the third section is set to 130°C, and the fourth section is set to 130°C. The section is set to 130°C, the head temperature is set to 130°C), the premix of drug and carrier is added, and at a speed of 36 rpm, the melt extruder shears and disperses it, and melts and extrudes it at room temperature After cooling or quenching, a golden yellow transparent solid solution is obtained.
[0019] (2) The preparation of nimodipine solid solution dispersible tablets: get 200 grams of nimodipine solid solution, 73 grams of microcrystalline cellulose, 15 grams of crospovidone, 3 grams of sodium lau...
Embodiment 2
[0022] (1) Preparation of nimodipine solid solution: take polyethylene glycol as the carrier, premix with nimodipine according to the ratio of 9:1 and 5 minutes, and the temperature of the melt extruder from the first section to the machine head is respectively Set at 90°C-130°C-130°C-130°C-130°C, add the premix of drug and carrier, and at a speed of 36 rpm, the melt extruder shears and disperses it, and melts and extrudes it. After cooling at room temperature or quenching, a golden yellow transparent solid solution is obtained.
[0023] (2) Preparation of nimodipine solid solution dispersible tablets: get 300 grams of nimodipine solid solution, 108 grams of starch, 27 grams of croscarmellose sodium, 4.5 grams of poloxamer and 10.5 grams of magnesium stearate, Nimodipine solid solution is pulverized, 60-80 mesh particles are collected, starch is made into soft material with 8% starch slurry, granulated with a 60 mesh sieve, granulated with a 60 mesh sieve after drying, mixed w...
Embodiment 3
[0026] (1) Preparation of nimodipine solid solution: take Acrylic Resin No. 4 as the carrier, premix with nimodipine according to the ratio of 5:1 and 5 minutes, and melt the extrusion machine from the first section to the temperature of the head Set at 90°C-130°C-130°C-130°C-130°C respectively, add the premix of drug and carrier, and at a speed of 36 rpm, the melt extruder shears and disperses it, and melts and extrudes it , After cooling at room temperature or quenching, a golden yellow transparent solid solution is obtained.
[0027] (2) The preparation of nimodipine solid solution dispersible tablets: get 180 grams of nimodipine solid solution, 65 grams of dextrin, 30 grams of low-substituted hydroxypropyl cellulose, 15 grams of sodium lauryl sulfate and 10 grams of micropowder silica gel, Nimodipine solid solution is pulverized, 60-80 mesh particles are collected, 8% starch slurry is used as soft material for dextrin, granulated with 60 mesh sieve, granulated with 60 mesh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com