Nimodipine micelle injection and preparation method thereof
A technology of nimodipine glue and micelle injection, which is applied in the field of medicine, can solve the problems of increasing the pain of patients and the mental burden of medical workers, not fundamentally solving the problems of serious side effects of organic solvents, increasing treatment costs, etc., and achieving convenient clinical administration. Drug, improve water solubility and stability, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Weigh 50 mg of egg yolk lecithin, 50 mg of sodium glycocholate, and 1.25 mg of nimodipine into a 50 ml round bottom flask, add 10 ml of ethanol to dissolve, and disperse evenly by ultrasonication. The ethanol was removed by rotary evaporation at a water bath temperature of 40° C. until there was no alcohol smell and a layer of transparent film was formed, and then dispersed with 2.5 ml of water for injection to obtain a dispersion solution of drug-containing mixed micelles. After adding 0.05% activated carbon for injection and stirring for 15 minutes, centrifuge at 12000rpm / min for 5 minutes, then filter with a 0.22μm microporous membrane, take the filtrate, subpackage, and sterilize by autoclaving at 121°C for 15 minutes to obtain nimodipine mixed gel Bundle of injections.
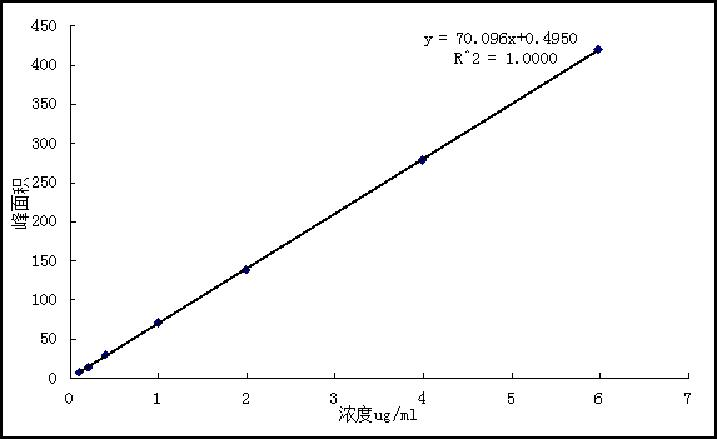
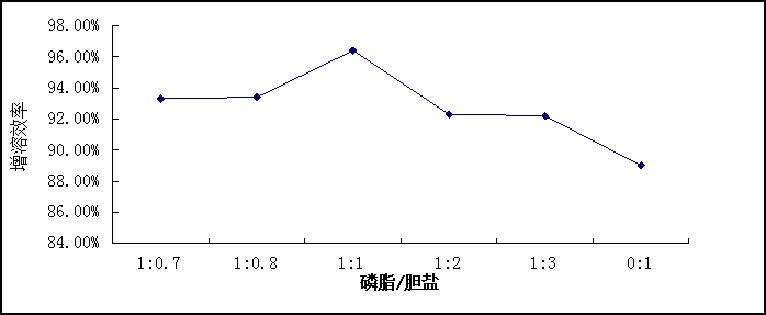
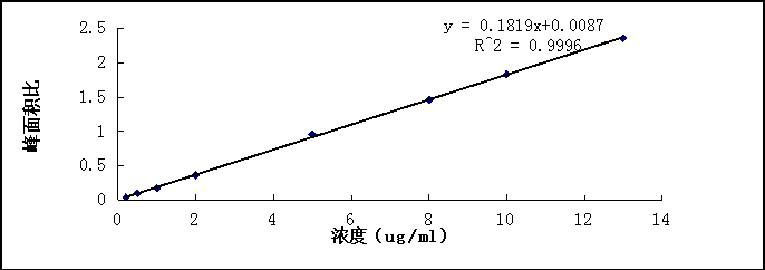
[0060] The solubilization efficiency measured according to Example 5 below was 96%, the concentration of nimodipine was 0.48 mg / ml, and the drug loading was 1.2%.
[0061]
Embodiment 2
[0063] Weigh 50 mg of egg yolk lecithin, 50 mg of sodium glycocholate, and 1 mg of nimodipine into a 50 ml round bottom flask, add 10 ml of ethanol to dissolve, and disperse evenly by ultrasonication. Then spin evaporate to remove ethanol, the water bath temperature is 35 ℃, spin to no alcohol smell, form a layer of transparent film, then disperse with 2.5ml of water for injection, obtain the dispersion solution containing drug mixed micelles. After adding 0.05% activated carbon for injection and stirring for 15 minutes, centrifuge at 12000rpm / min for 5 minutes, then filter with a 0.22μm microporous membrane, take the filtrate, subpackage, and sterilize by autoclaving at 121°C for 15 minutes to obtain nimodipine mixed gel Bundle of injections.
[0064] The solubilization efficiency measured according to Example 5 below was 93%, the concentration of nimodipine was 0.37 mg / ml, and the drug loading was 0.9%.
[0065]
Embodiment 3
[0067] Weigh 45mg of polyene phosphatidylcholine, 40mg of sodium deoxycholate, and 1mg of nimodipine into a 50ml round bottom flask, add 10ml of ether, dissolve, and disperse evenly by ultrasonication. At a water bath temperature of 35° C., diethyl ether was removed by rotary evaporation to form a transparent film, which was then dispersed with 2.5 ml of water for injection to obtain a dispersion solution containing drug-mixed micelles. After adding 0.05% activated carbon for injection and stirring for 15 minutes, centrifuge at 12000rpm / min for 5 minutes, then filter with a 0.22μm microporous membrane, take the filtrate, subpackage, and sterilize by autoclaving at 121°C for 15 minutes to obtain nimodipine mixed gel Bundle of injections.
[0068] According to Example 5 below, the solubilization efficiency was 90%, the concentration of nimodipine was 0.36 mg / ml, and the drug loading was 1.0%.
[0069]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com