Nimodipine sub micro-emulsion injection and preparation method thereof
A technology of nimodipine and nimodipine, which is applied in the field of medicine, can solve the problems of patient inconvenience and reinforcement, and achieve the effects of reducing workload, improving compliance, and avoiding drug precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
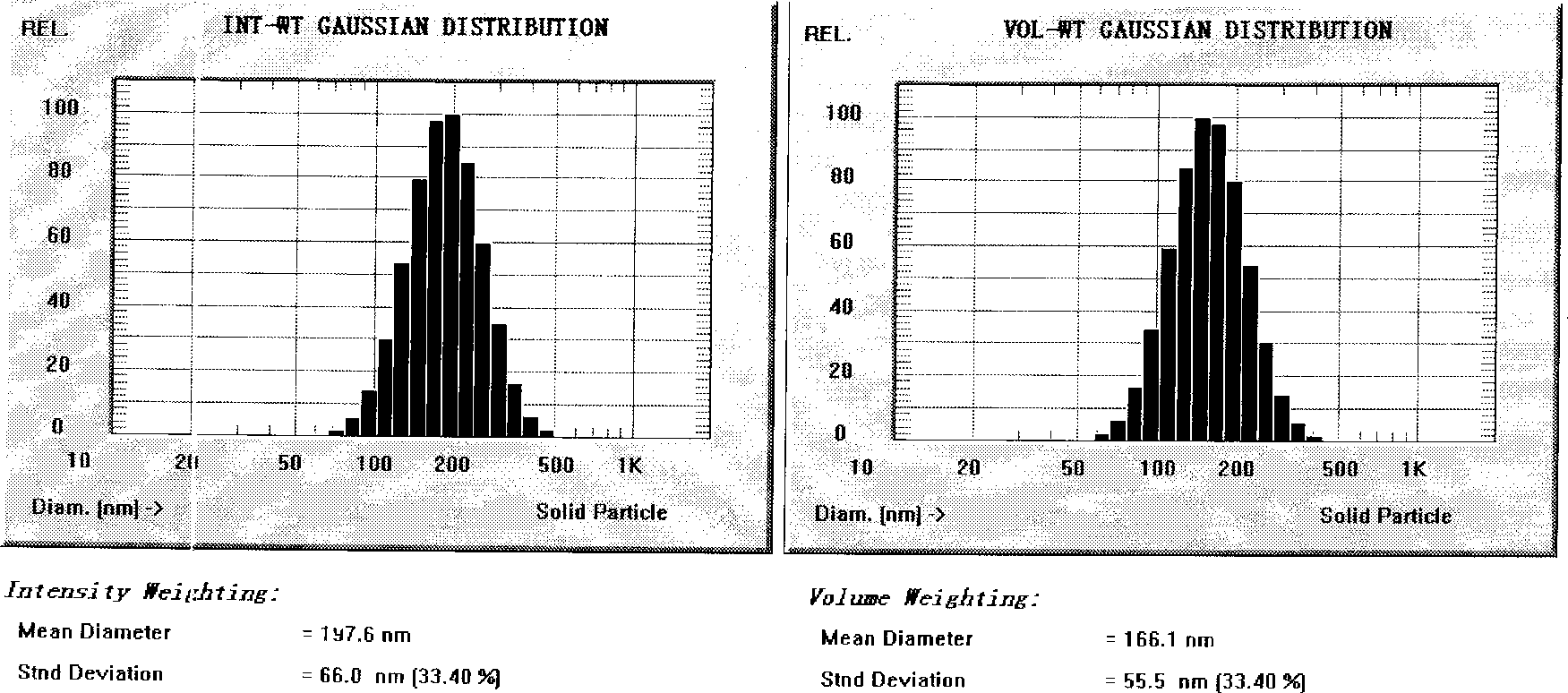
[0039] (1) Under the protection of nitrogen, weigh 1.0 g of nimodipine, 80 g of soybean oil, 25 g of soybean lecithin, 1.0 g of oleic acid, and 0.5 g of α-tocopherol, and stir and dissolve in a water bath at 65° C. as the oil phase; ( 2) Weigh 15 g of glycerin and add it to an appropriate amount of water for injection, and dissolve it in a water bath at 65° C. as the water phase. (3) Drop the water phase into the oil phase under stirring, cut it into colostrum by high-speed shearing machine, adjust the pH to 5.5 with 0.2% citric acid, add water for injection to 1000mL; Circulate the emulsion 6 times to reach the specified particle size, filter and sterilize through a 0.22 μm filter membrane, fill with nitrogen and repackage, and sterilize to obtain nimodipine submicroemulsion injection with a particle size of 197.6 nm and a zeta potential value of -36 mv.
Embodiment 2
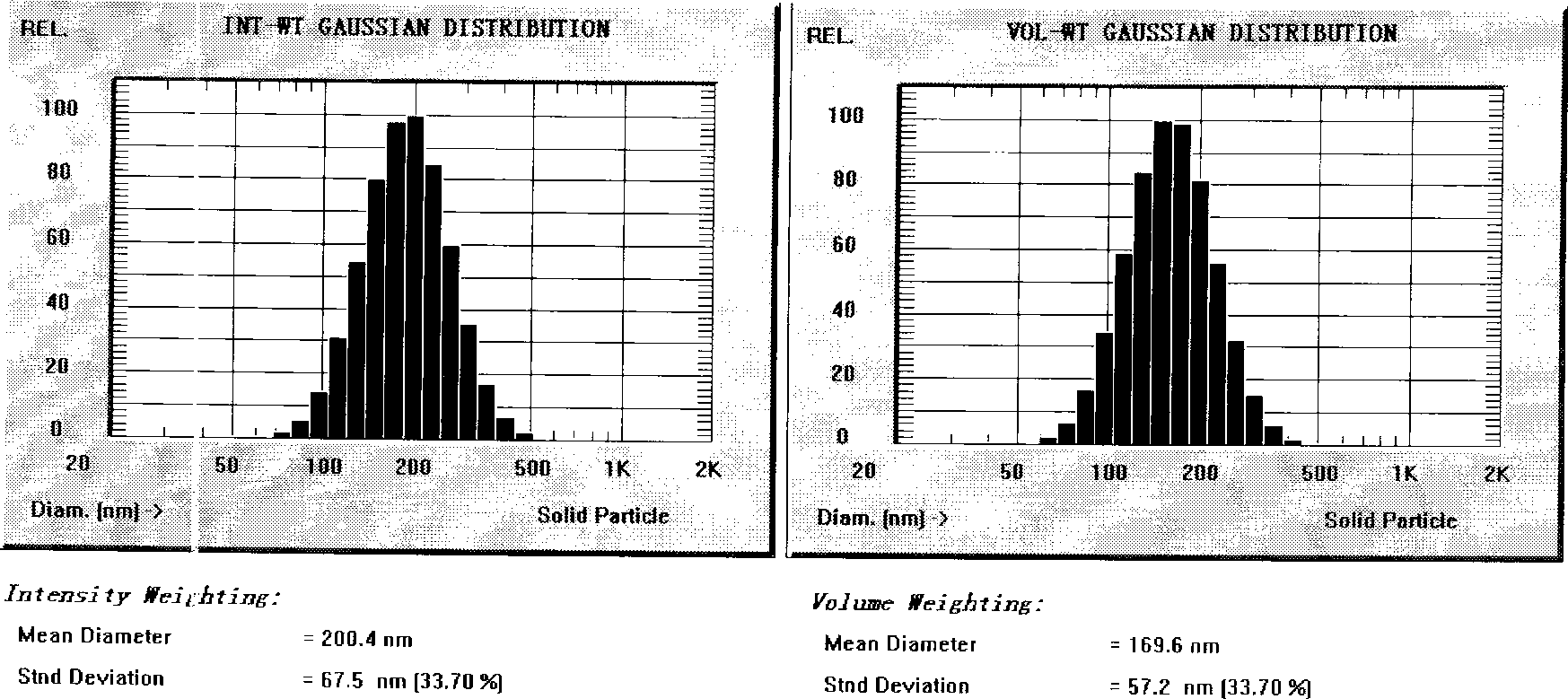
[0041] (1) Under nitrogen protection, weigh 1.5 g of nimodipine, 60 g of medium-chain triglycerides, 30 g of egg yolk phospholipids, 3.0 g of linoleic acid, and 1 g of tocopherol, and place them in a water bath at 70° C. for stirring and dissolving as an oil phase; (2) Weigh 5.0 g of poloxamer, add 20 g of sorbitol into an appropriate amount of water for injection, dissolve in a water bath at 70° C., and serve as the water phase. (3) Drop the water phase into the oil phase under stirring, and cut it into colostrum by high-speed shearing machine, adjust the pH to 5.0 with 0.5mol / L phosphoric acid, add water for injection to 1000mL; The machine circulates the emulsion 7 times until it reaches the specified particle size, and then it is sterilized by filtration through a 0.22 μm filter membrane, filled with nitrogen, and then sterilized to obtain nimodipine submicroemulsion injection with a particle size of 200.4nm and a zeta potential value of -25mv .
Embodiment 3
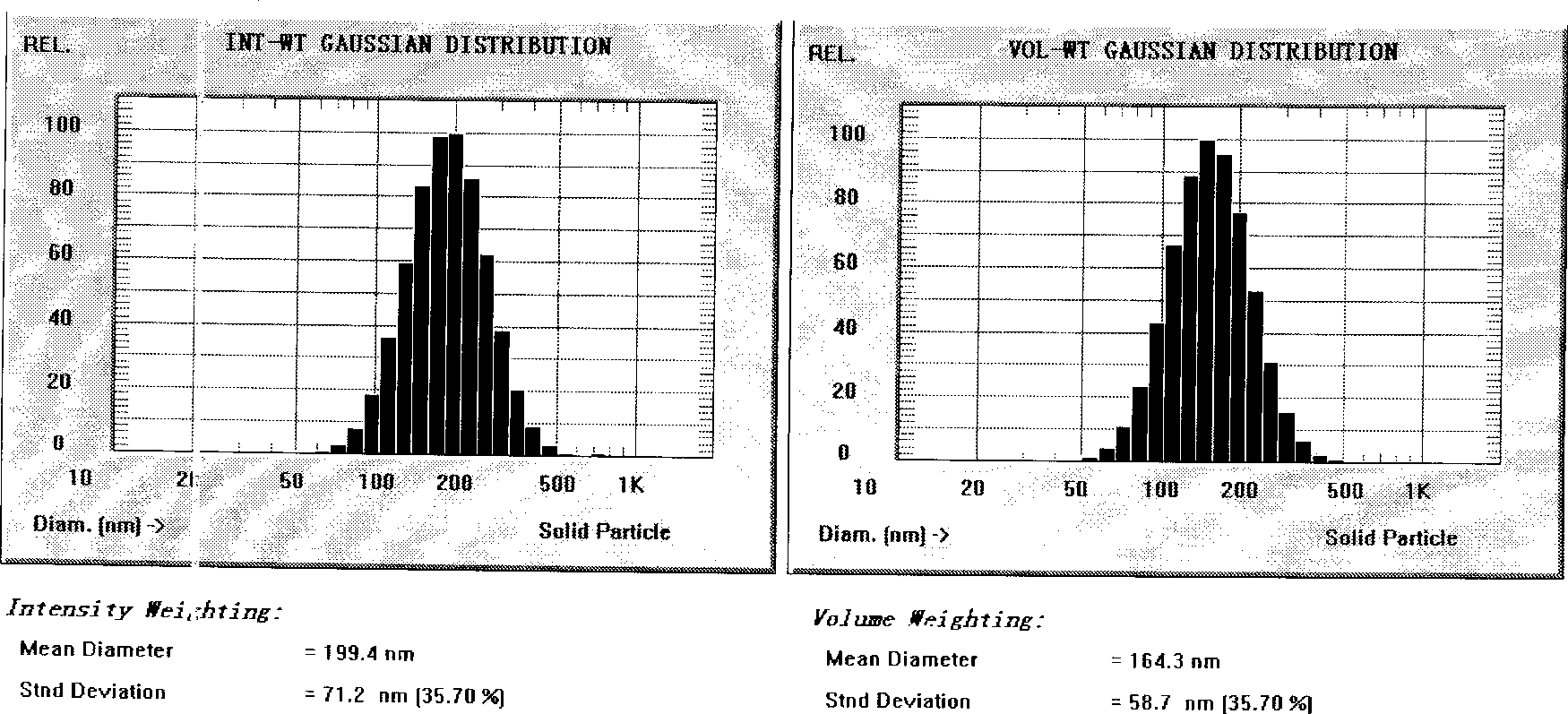
[0043] (1) Under the protection of nitrogen, weigh 3.0 g of nimodipine, 100 g of olive oil, and 45 g of egg yolk phospholipids, place them in a water bath at 75°C and stir to dissolve them as the oil phase; (2) weigh 2.0 g of sodium oleate and 2.0 g of sodium sulfite g, adding 60 g of glycerin into water for injection, and dissolving in a water bath at 75° C. as the water phase. (3) Drop the water phase into the oil phase under stirring, cut it into colostrum by high-speed shearing machine, adjust the pH to 7.0 with 0.1mol / L NaOH solution, add water for injection to 1000mL; The machine circulates the emulsion 8 times until the specified particle size is reached, and is sterilized by filtration through a 0.22 μm filter membrane, filled with nitrogen, subpackaged, and sterilized to obtain nimodipine submicroemulsion injection with a particle size of 199.4nm and a zeta potential value of -32mv .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com