Nimodipine solid dispersoid and preparation method thereof
A nimodipine solid, nimodipine technology, applied in the field of medicine, can solve the problems of aging, slow drug dissolution, insoluble tablets and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
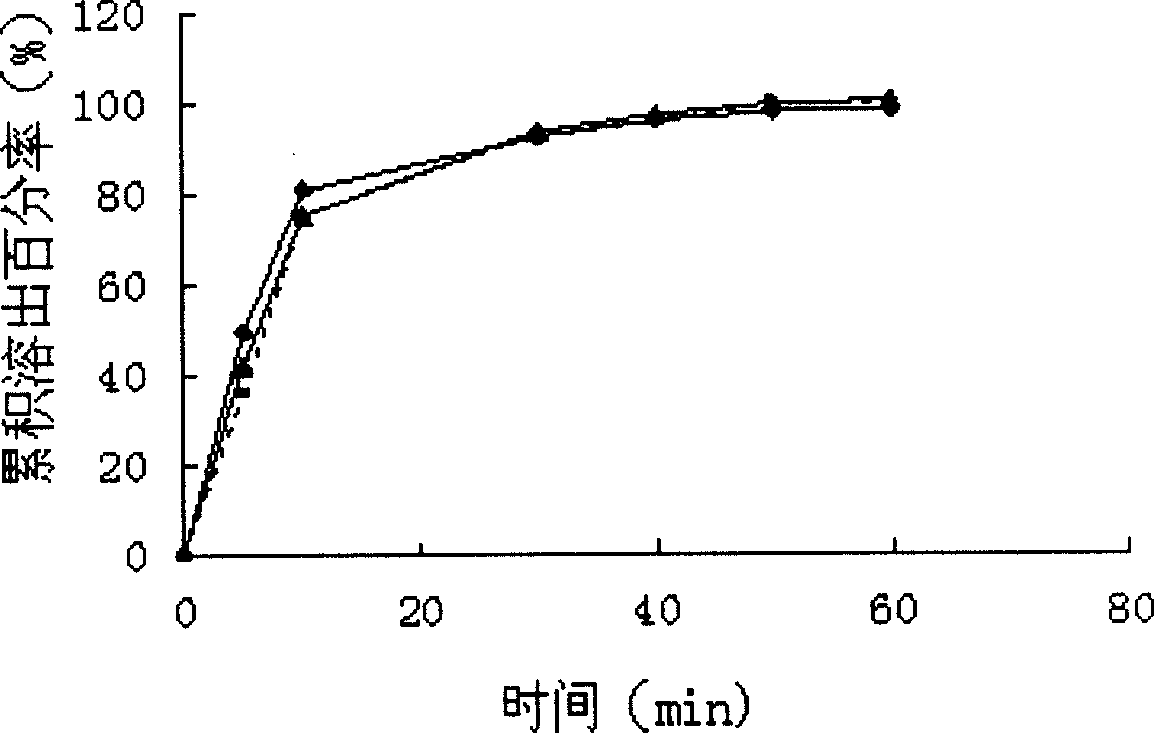
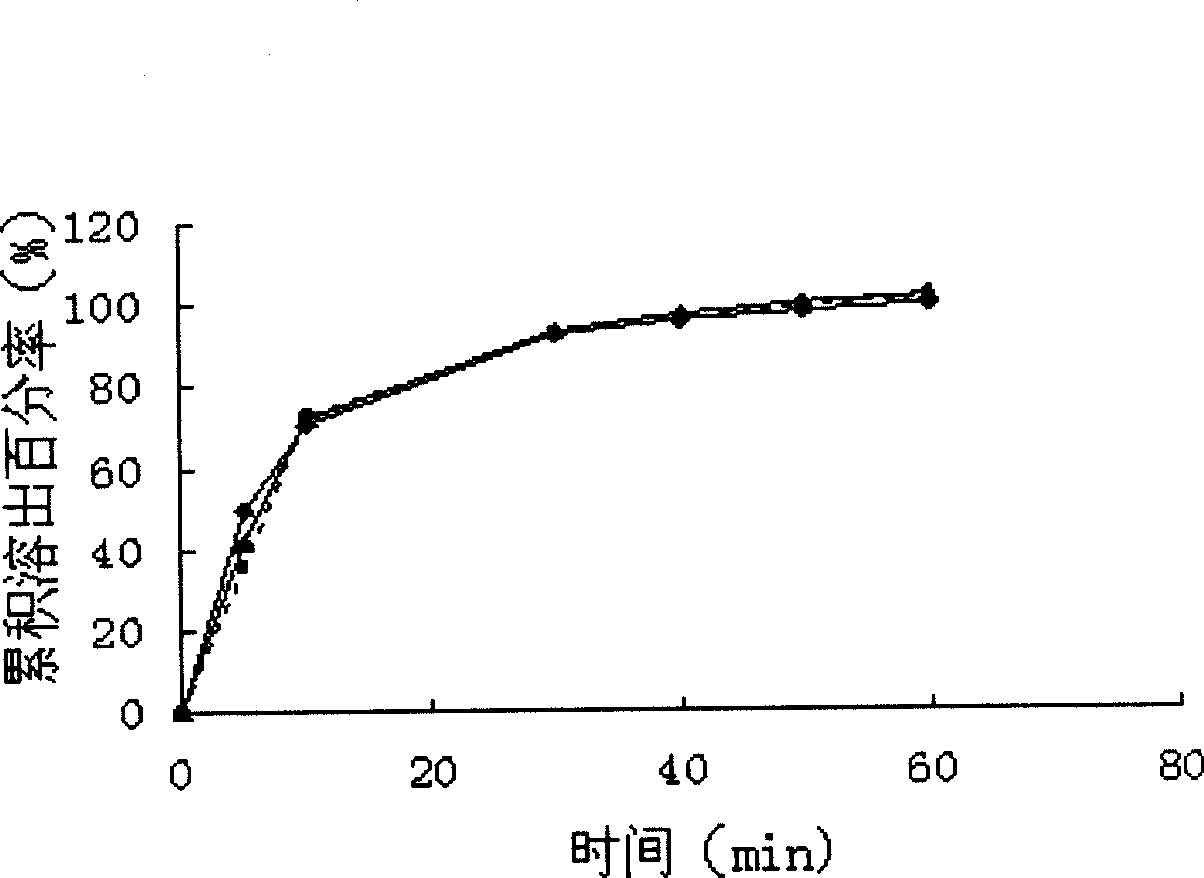
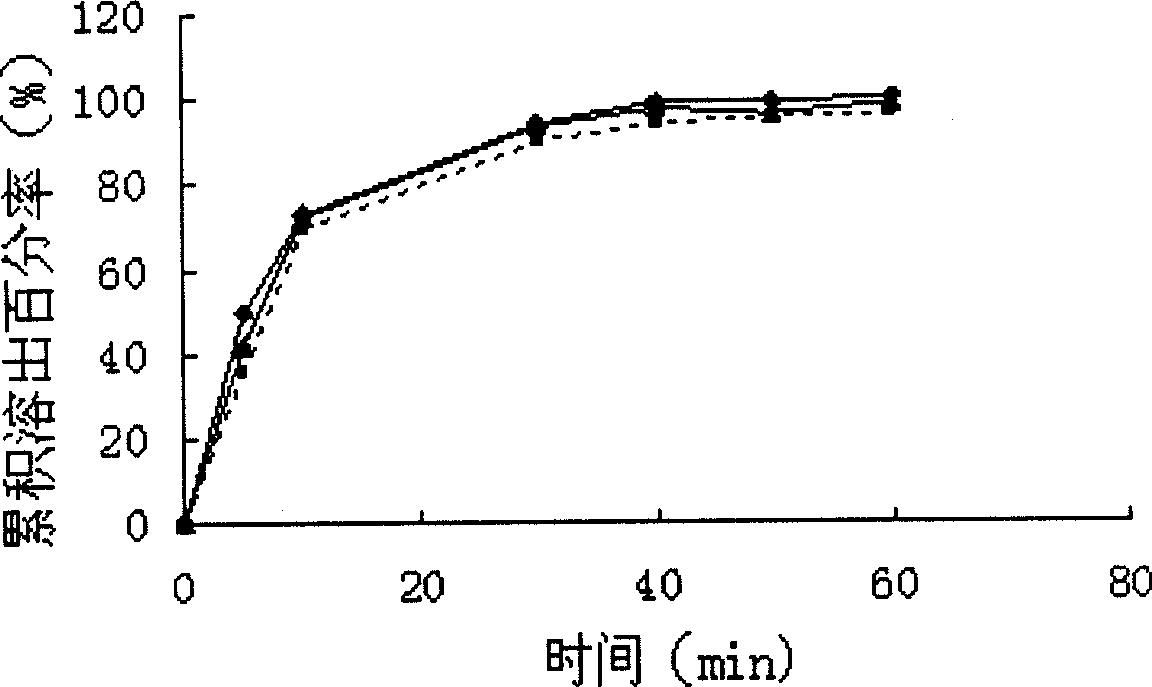
[0041] Take 10g of PEG4000 and 0.5g of micro-powdered silica gel and mix well, heat to melt PEG4000, add 1g of nimodipine, mix well, quickly pour the molten material on the steel plate and spread it flat, put it in a -20°C refrigerator, freeze for 8 hours, and then transfer to a vacuum Continue to dry in the drying oven for 24-48h (at room temperature), take it out, grind it, and pass it through an 80-mesh sieve. The solid dispersion of the invention has an in vitro cumulative dissolution percentage of 101.12±2.45% (n=6) within 45 minutes.
Embodiment 2
[0043] Take 5g of PEG4000 and 5g of micro-powder silica gel and mix well, heat to melt PEG4000, add 5g of nimodipine, mix well, quickly pour the molten material on the steel plate, place in -20°C refrigerator, freeze for 8 hours, and then transfer to vacuum drying Continue to dry in the box for 24-48h (at room temperature), take it out, grind it, and pass it through an 80-mesh sieve. The solid dispersion of the invention has an in vitro cumulative dissolution percentage of 100.16±1.30% (n=6) within 45 minutes.
Embodiment 3
[0045] Take 10g of PEG4000 and 0.5g of talcum powder and mix well, heat to melt PEG4000, add 1g of nimodipine, mix well, quickly pour the molten material on the steel plate and spread it, put it in -20℃ refrigerator, freeze for 8 hours, and then transfer to vacuum Continue to dry in the drying oven for 24-48h (at room temperature), take it out, grind it, and pass it through an 80-mesh sieve. The solid dispersion of the invention has an in vitro cumulative dissolution rate of 99.22±2.05% (n=6) within 45 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com