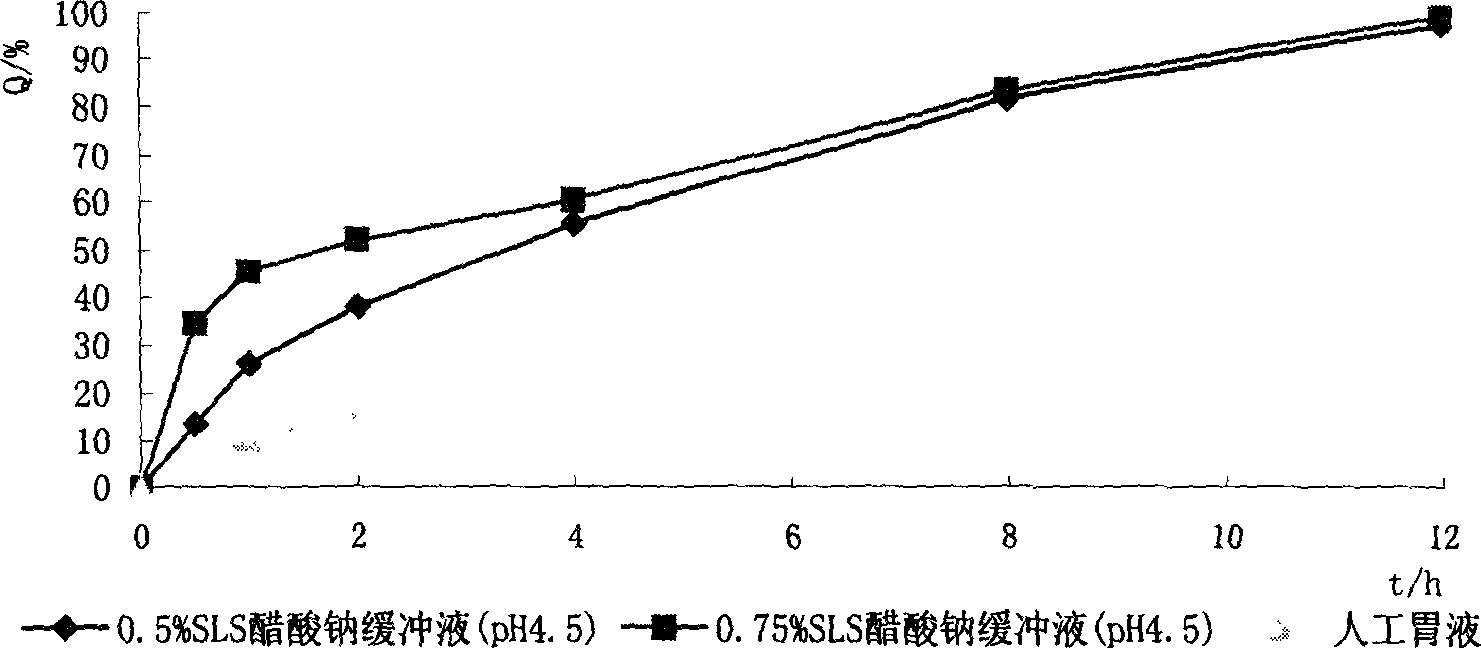
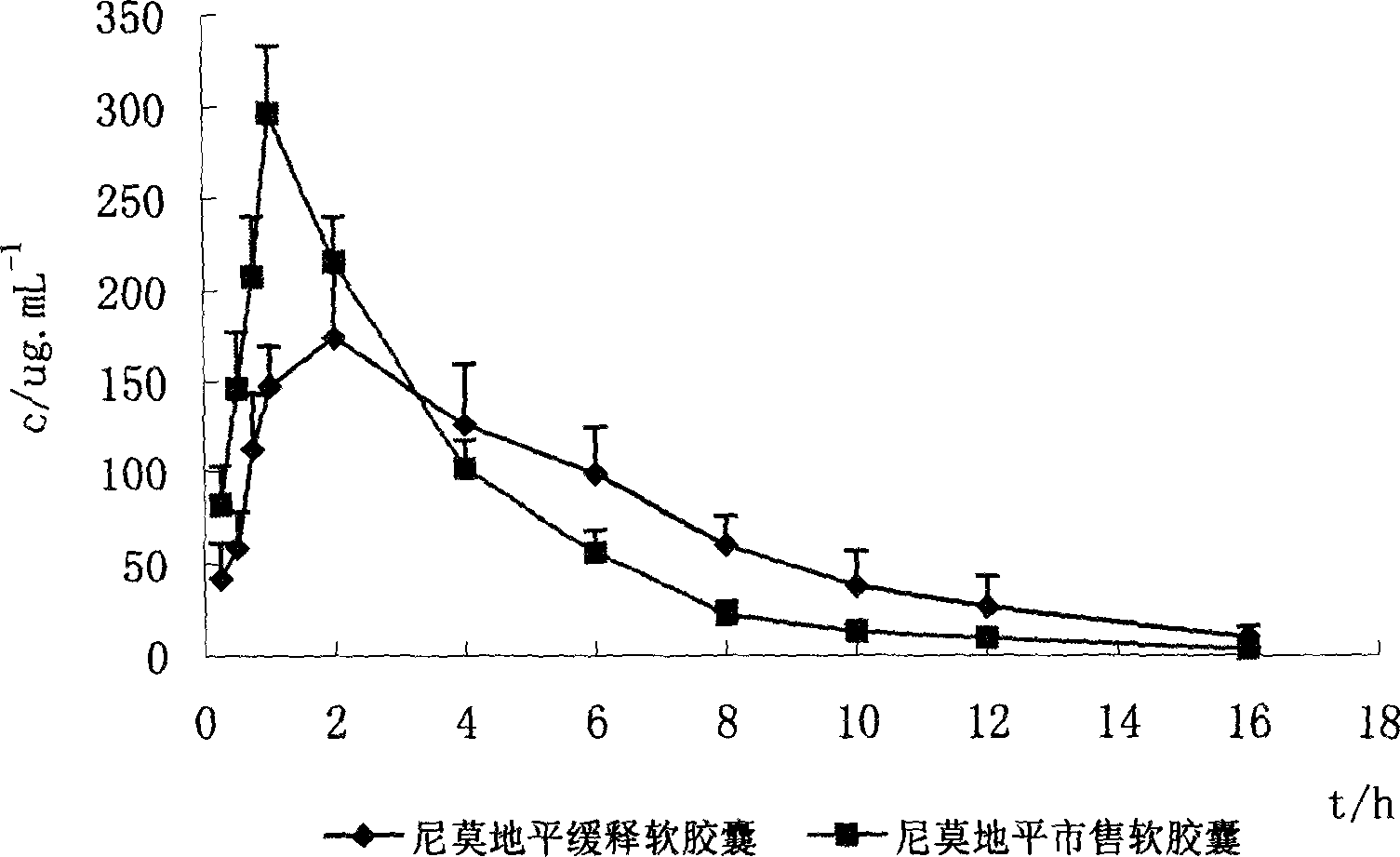
Nimodipine soft capsule and its prepn
A technology for nimodipine and soft capsules, applied in the field of nimodipine sustained-release soft capsules and its preparation, can solve the problems of low bioavailability, large blood concentration fluctuations, and many times of taking, and achieve the effect of simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of contents: Take 400g of nimodipine, 3000g of PEG400, 1000g of PEG 600, and 100g of Tween 80 in a liquid mixing tank and heat and stir through interlayer steam until all nimodipine is dissolved to obtain liquid A. Take 20g of sodium alginate and 20g of pectin, dissolve them in 1000g of water, swell for half an hour, heat and stir to dissolve to obtain liquid B, add liquid A to liquid B, and mix well to obtain the content solution.
[0065] Capsule material preparation: Weigh 3500g water, 1155g glycerin, 3500g gelatin, 20.5g titanium dioxide, stir into uniform particles, add 75g 20% paraben ethanol solution and 63g calcium chloride to carry out sol.
[0066] Pressed pills: Pressed on a soft capsule machine, and then shaped, washed and dried.
Embodiment 2
[0068] Contents preparation: take 20g sodium alginate, 10g gum arabic and 15g carrageenan, dissolve them in 800g water, swell for half an hour, heat and stir to dissolve; add 3200g PEG400 and 400g nimodipine in sequence and pass through the interlayer steam in the liquid preparation tank Heat and stir until a homogeneous mixture is formed, pass through a high-pressure milk homogenizer several times, and keep warm for later use.
[0069] Capsule material preparation: Weigh 3250g water, 1400g glycerin, 3500g gelatin, 16.5g titanium dioxide, stir into uniform particles, add 52.5g 20% paraben ethanol solution and 94.5g calcium tartrate to carry out sol.
[0070] Pressed pills: Pressed on a soft capsule machine, and then shaped, washed and dried.
Embodiment 3
[0072] Contents preparation: Take 30g pectin and 10g gum arabic, dissolve them in 1200g water, swell for half an hour, heat and stir to dissolve; add 4000g PEG400 and 400g nimodipine in sequence, heat and stir through interlayer steam in the liquid mixing tank until a uniform mixture is formed , through a high-pressure milk homogenizer several times, and keep warm for later use.
[0073] Capsule material preparation: Weigh 3500g water, 1750g glycerin, 3500g gelatin, 87.5g iron oxide, stir into uniform granules, add 35g 20% paraben ethanol solution, 120g sodium citrate and 31.5g calcium chloride to carry out sol.
[0074] Pressed pills: Pressed on a soft capsule machine, and then shaped, washed and dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com