Fat emulsion injection containing nimodipine and preparation method thereof
A technology of nimodipine fat and nimodipine, which is applied in the field of medicine, can solve the problems of patients' inability to tolerate side effects, lower drug safety for patients, and increase the risk of venous inflammation, etc., so as to solve the problems of easy drug precipitation, safety and biophase The effect of good capacitance and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
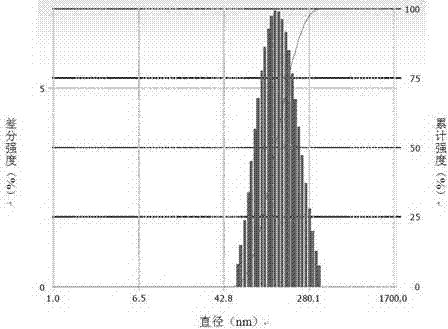
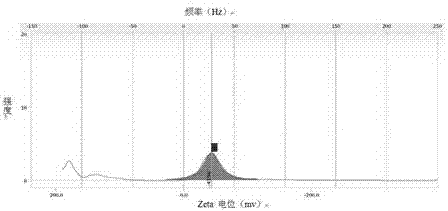
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of nimodipine fat emulsion injection
[0037] (1) The formulation ratio of nimodipine fat emulsion injection is shown in the following table: (made into 1000ml)
[0038] components Weight to volume ratio (%) Feeding amount Nimodipine 0.08 0.8g Soybean Oil for Injection 10.00 100g Yolk Lecithin for Injection 1.00 10g Lithium Polyethylene Glycol Lauryl Hydroxystearate 0.20 2g sodium hydroxide Appropriate amount Adjust to pH 9.0 Sorbitol 2.25 22.5g Water for Injection Appropriate amount Add to 1000ml
[0039] (2) The preparation method of nimodipine fat emulsion injection is as follows: a. Under the protection of nitrogen, weigh the prescribed amount of nimodipine and oil for injection, ultrasonically disperse the drug in the oil for 3 minutes, and then add the prescribed amount for injection Use phospholipids and polyethylene glycol lauryl hydroxystearate, stir in a 60°C w...
Embodiment 2
[0040] Embodiment 2: the preparation of nimodipine fat emulsion injection
[0041] (1) The formulation ratio of nimodipine fat emulsion injection is shown in the following table: (made into 1000ml)
[0042] components Weight to volume ratio (%) Feeding amount Nimodipine 0.08 0.8g Soybean Oil for Injection 10.00 100g Soybean Lecithin for Injection 1.20 12g Lithium Polyethylene Glycol Lauryl Hydroxystearate 0.20 2g Potassium hydroxide Appropriate amount Adjust to pH 8.0 Sorbitol 2.25 22.5g Water for Injection Appropriate amount Add to 1000ml
[0043](2) The preparation method of nimodipine fat emulsion injection is as follows: a. Under the protection of nitrogen, weigh the prescribed amount of nimodipine and oil for injection, ultrasonically disperse the drug in the oil for 3 minutes, and then add the prescribed amount for injection Use phospholipids and polyethylene glycol lauryl hydroxystearate, stir in a 6...
Embodiment 3
[0044] Embodiment 3: the preparation of nimodipine fat emulsion injection
[0045] (1) The formulation ratio of nimodipine fat emulsion injection is shown in the following table: (made into 1000ml)
[0046] components Weight to volume ratio (%) Feeding amount Nimodipine 0.08 0.8g Soybean Oil for Injection 5.00 50g Medium Chain Triglycerides for Injection 5.00 50g Yolk Lecithin for Injection 1.00 10g Lithium Polyethylene Glycol Lauryl Hydroxystearate 0.20 2g Citric Acid / Sodium Citrate Appropriate amount Adjust to pH 5.0 glycerin 2.25 22.5g Water for Injection Appropriate amount Add to 1000ml
[0047] (2) The preparation method of nimodipine fat emulsion injection is as follows: a. Under the protection of nitrogen, weigh the prescribed amount of nimodipine and oil for injection, ultrasonically disperse the drug in the oil for 3 minutes, and then add the prescribed amount for injection Use phospholipids...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com