PirB extracellular polypeptide and application
A sequence and drug technology, applied in the field of bioengineering protein recombination, can solve the problem of no effective therapeutic drugs and achieve the effect of promoting axon growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Principles and Ideas of Recombining PEP
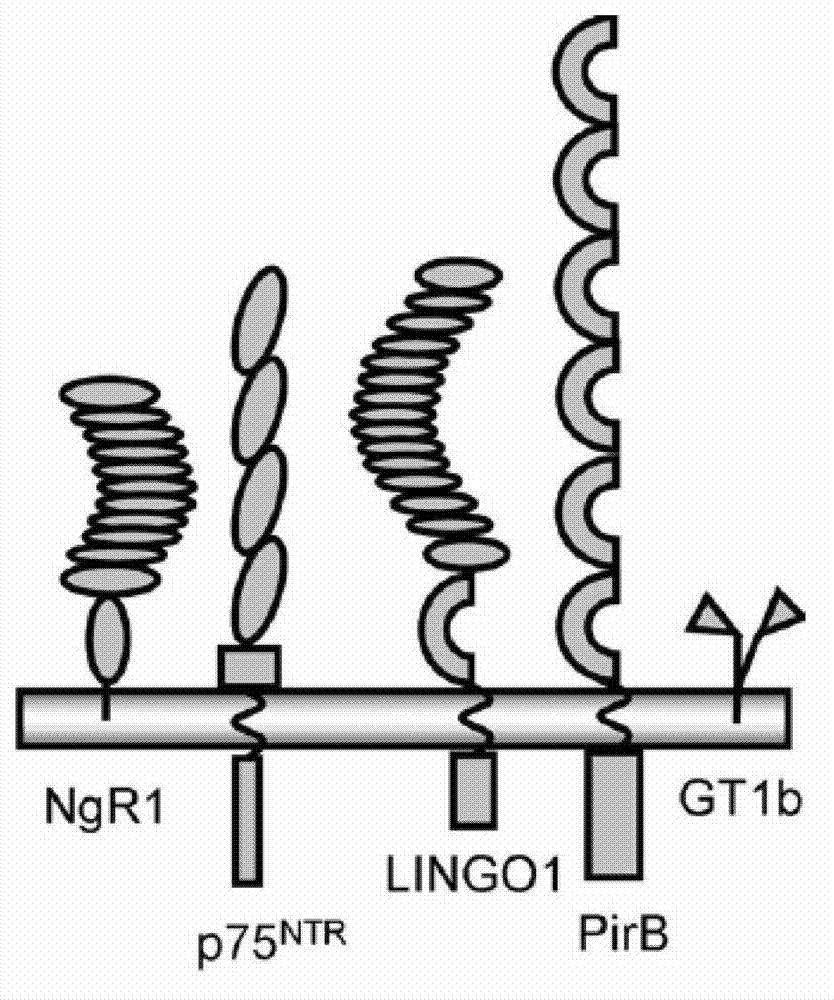
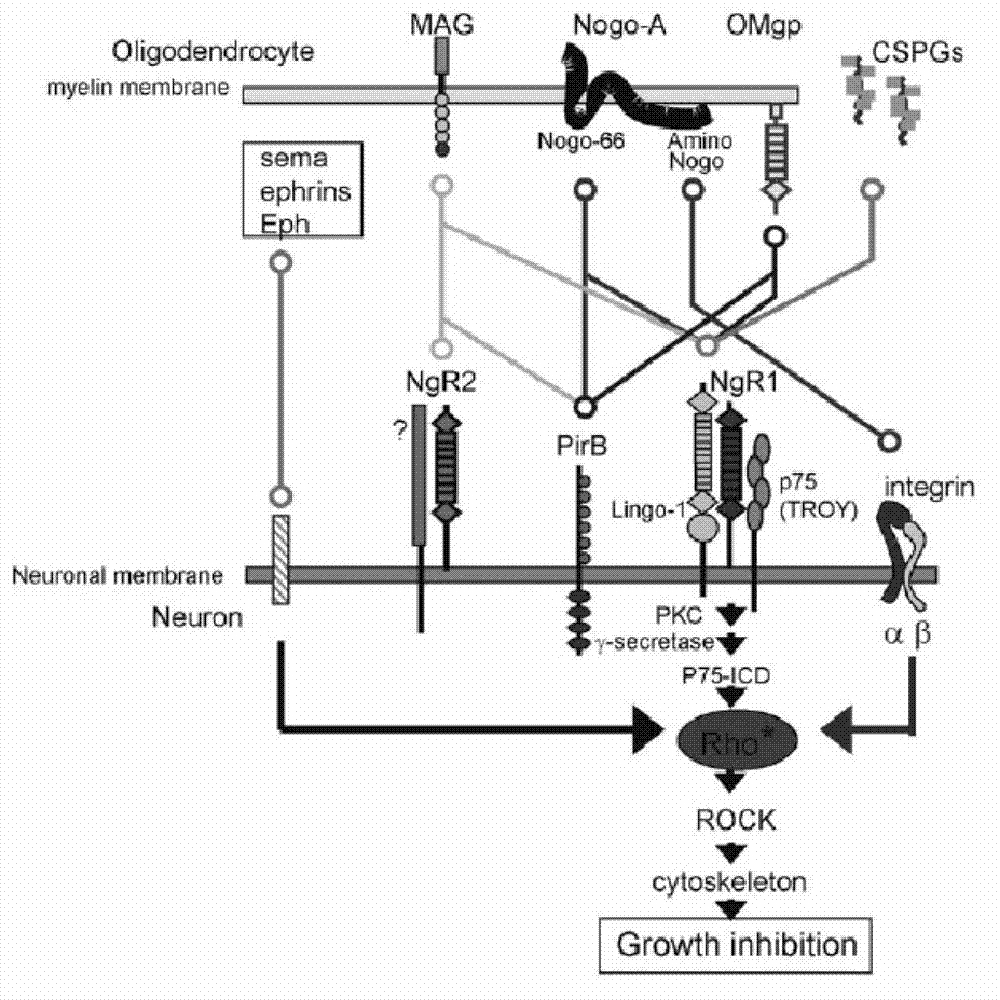
[0024] In 2008, Atwal et al. first reported that PirB is another functional receptor of myelin inhibitory factor in "Science". Later, some scholars published a review in "Neuron" and others, and believed that the discovery of PirB as a myelin receptor is a clinically promoted axis. Synaptic regeneration and neurological recovery provide a new therapeutic target. PirB is a type 1 transmembrane glycoprotein consisting of six extracellular immunoglobulin-like domains and intracellular polypeptides containing four immunoreceptor tyrosine-dependent inhibitory motifs (ITIMs) ( figure 1) . Relying on library screening technology, Atwal et al. found that PirB is a functional receptor of myelin protein, which can bind with MAG, Nogo-66, Omgp with high affinity ( figure 2) . Compared with NgR1, PirB appears to be more important in myelin inhibition, on the basis that genetic knockout of PirB causes more axonal regeneration...
Embodiment 2
[0025] Example 2: PEP expression, identification and purification
[0026] 1. Reagents and materials
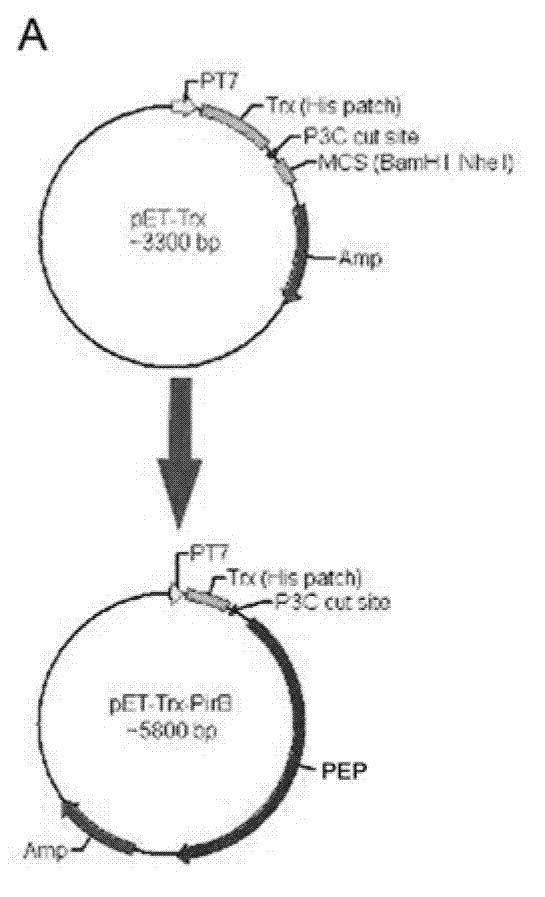
[0027] (1). Plasmids, strains
[0028] Strain: BL21(DE3)
[0029] Plasmid: pET32a
[0030] (2).Main reagents
[0031] Amp (ampicillin)
[0032] TPTG (isopropyl-β-D-thiogalactoside)
[0033] PBS (phosphate buffered saline)
[0034] SDS (Sodium Dodecyl Sulfate)
[0035] LB Borth Medium
[0036] LB Ager Medium
[0037] 2. According to the molecular structure of PirB ( figure 1) And bioinformatics, relying on library screening technology to determine the amino acid sequence of PirB extracellular polypeptide.
[0038] The amino acid sequence of PirB extracellular polypeptide PEP is:
[0039] GSLPKPILRVQPDSVVSRRTKVTFLCEETIGANEYRLYKDGKLYKTVTKNKQKPENKAEFSFSNVDLSNAGQYRCSYSTQYKSSGYSDLLELVVTGHYWTPSLLAQASPVVTSGGYVTLQCESWHNDHKFILTVEGPQKLSWTQDSQYNYSTRKYHALFSVGPVTPNQRWICRCYSYDRNRPYVWSPPSESVELLVSGNLQKPTIKAEPGSVITSKRAMTIWCQGNLDAEVYFLHNEKSQKTQSTQTLQEPGNKGKFFIPSVTLQHAGQYRCYCYGSAGWSQ...
Embodiment 3
[0055] Embodiment three: PEP efficacy test
[0056] 1. Effects of different concentrations of PEP on the viability of primary neurons
[0057] (1). The hippocampal neurons were cultured until the fifth day, and different concentrations of purified fusion protein PEP were added, the concentrations were 62, 5 μg / L, 125 μg / L, and 250 μg / L;
[0058] (2). Normal culture, cell viability analysis (MTT method) at 12h, 24h, 48h after adding protein;
[0059] (3). The results showed that different concentrations of PEP protein had no effect on the viability of primary neurons, indicating that PEP had no toxicity to normal primary neurons.
[0060] 2. PEP promotes the growth of primary neuron axons and their branches and the formation of skeleton proteins
[0061] (1). Coating: Coat Nogo-66, MAG, OMgp(Sigma) (10mg / well) respectively in 96-well plates, incubate at 4°C for 24h, dry and wash with PBS (pH7.3 ) rinse 3 times;
[0062] (2). Blocking: add 2.5% calf serum (BSA) containing 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com