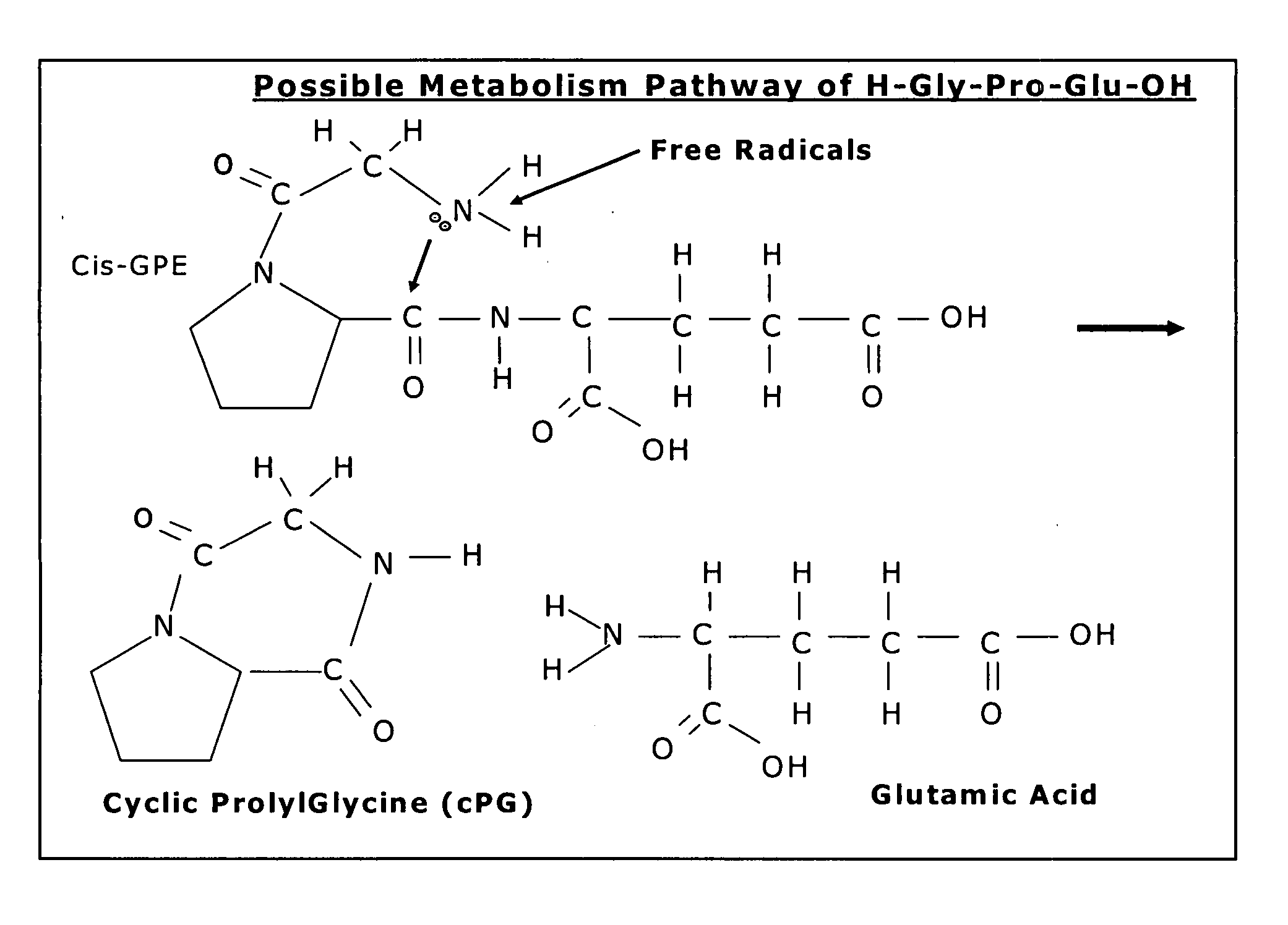
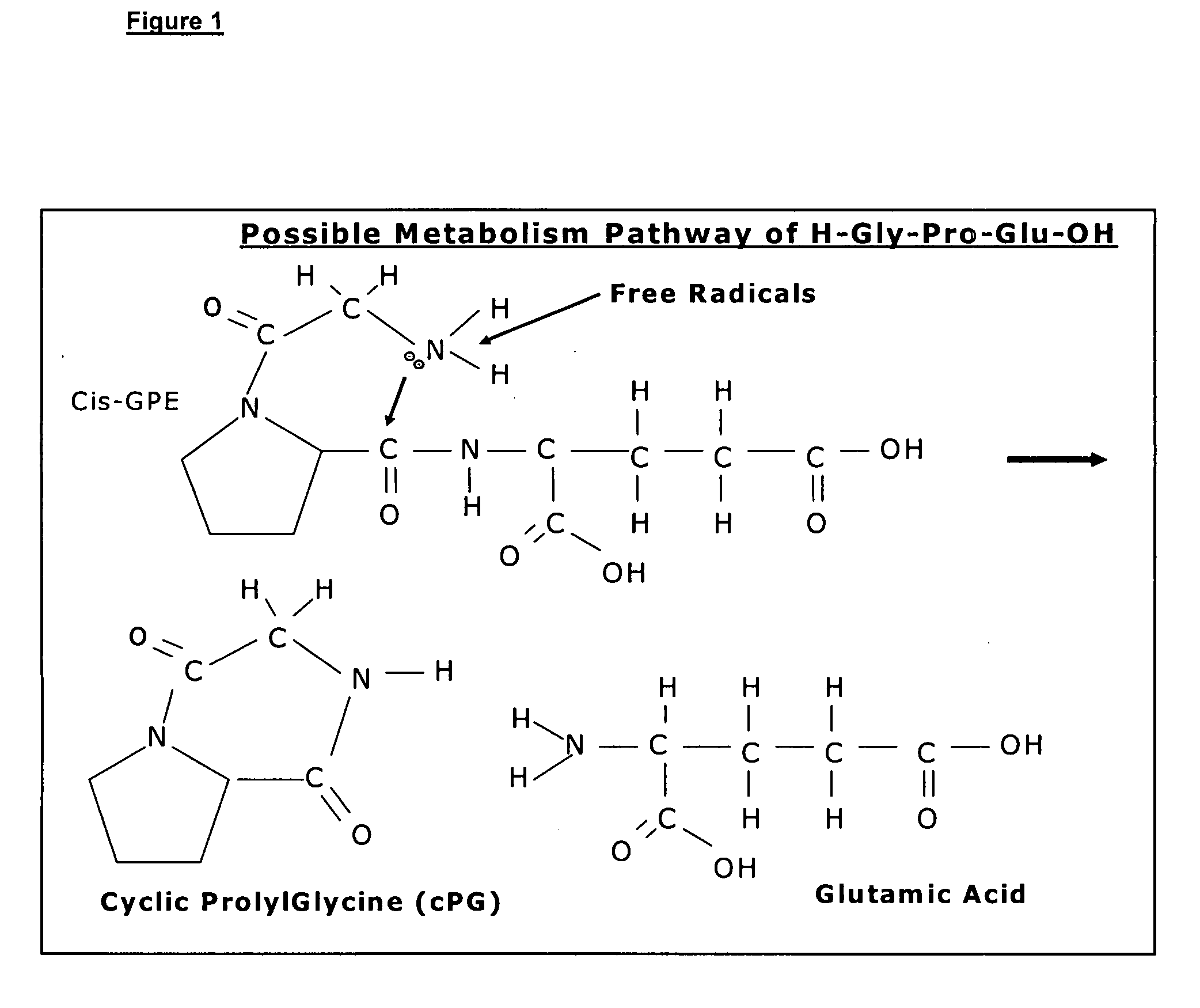
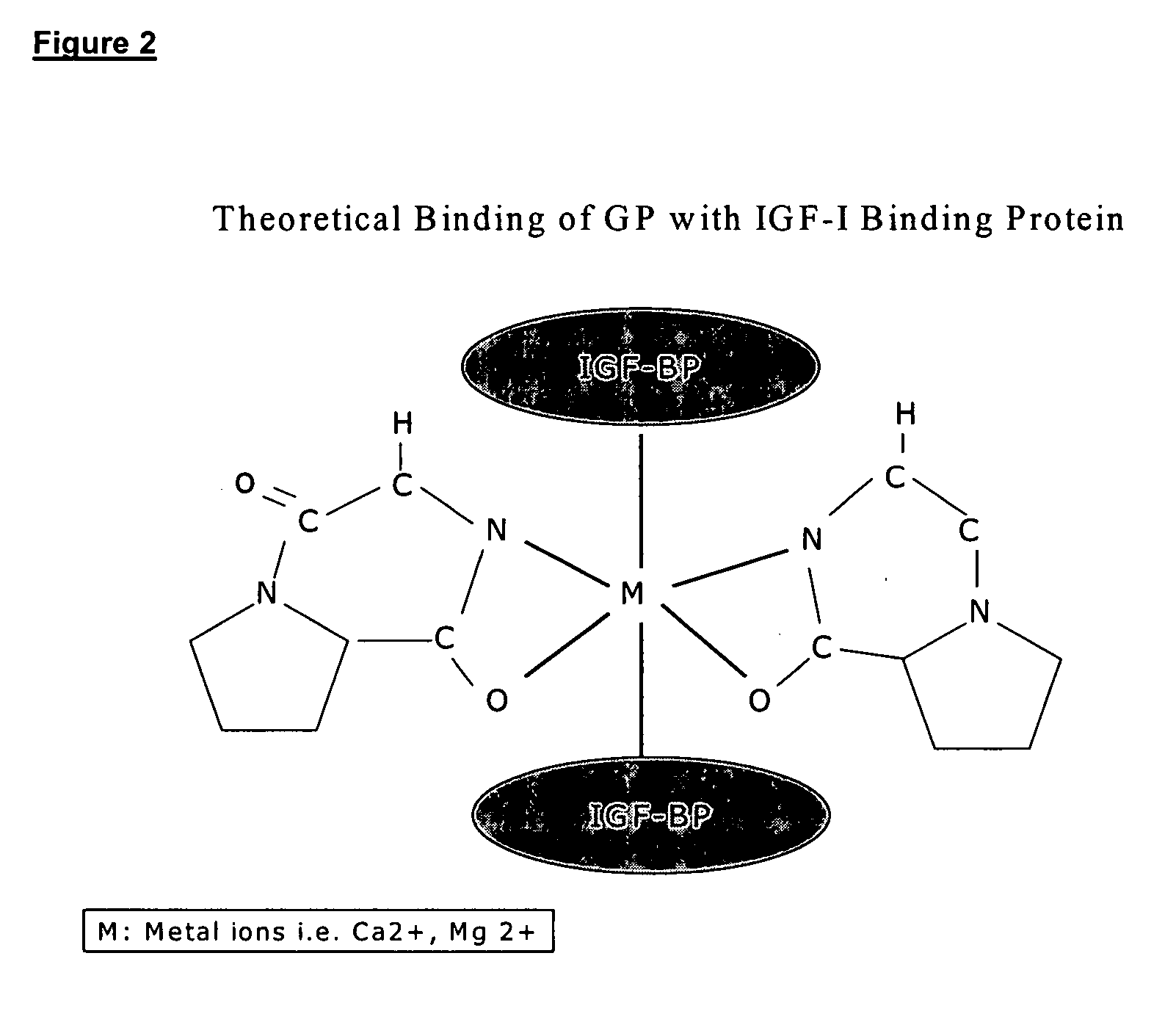
Therapeutic agent composition and method of use
a technology of therapeutic agents and compositions, applied in the field of nmda ampa receptors, can solve the problems of degeneration and death of neurons, difficult to determine the optimal dosage or human treatment, and the bell-shaped curve model of rats may be only applied to rats, and may not be entirely appropriate for human applications. to achieve the effect of promoting myelin production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1
[0094] Cyclic PG prevents glutamate induced neuronal death in vitro in a dose related manner.
Materials and Methods:
Cerebellar Cell Culture Preparing and coating of cover slips
[0095] Ten coverslips were placed into a large petri dish and washed in 70% alcohol for 5 minutes, then washed with Millipore H2O. The coverslips were air dried, then coated with Poly-D-Lysine (1 mg / ml stock solution in PBS, 90-100 μl) and incubated for 2 hours at 34° C.
Extraction
[0096] Postnatal day 4 Wistar rats were used for the study. Rats were placed in ice for 1 minute, the heads were decapitated and the cerebellum removed on ice. Cerebellum tissue was placed in 1 ml of 0.65% glucose supplemented PBS (10 μl 65% stock D (+)glucose / 1 ml PBS) in a large petri dish, chopped up into smaller sections and triturate with a 1 ml insulin syringe via a 23 G (0.4 mm) needle, and then squirted back into the glucose solution on the large petri dish. The tissue was sieved through (125 μm pore size gaze) and cent...
experiment 2
[0103] Effects of cPG after 6-OHDA induced nigral-striatal lesion.
Materials and Methods
[0104] Twenty male Wistar rats (280-310 g) were used. After exposing the skull, 6-OHDA (8 μg in a base of 2 μl 0.9% saline containing 1% ascorbic acid) was administered into the right medial forebrain bundle (MFB) using co-ordinates AP +4.7 mm, R 1.6 mmv-8 mm under 3% halothane anaesthesia. 6-OHDA was injected through a 25 G needle connected via a polyethylene catheter to a 100 μl Hamilton syringe. The 6-OHDA was infused by a microdialysis infusion pump at a rate of 0.5 μl / min. The needle was left in the brain for a further 3 minutes before being slowly withdrawn. The skin was sutured with 2.0 silk and the rats were allowed to recover from anaesthesia. The rats were housed in a holding room with free access to food and water at all times except during behavioural testing.
[0105] Cyclic PG was dissolved in saline. Four different doses of cPG (0, 0.1 0.5 1 mg / kg, Bachem) were administered intrape...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com