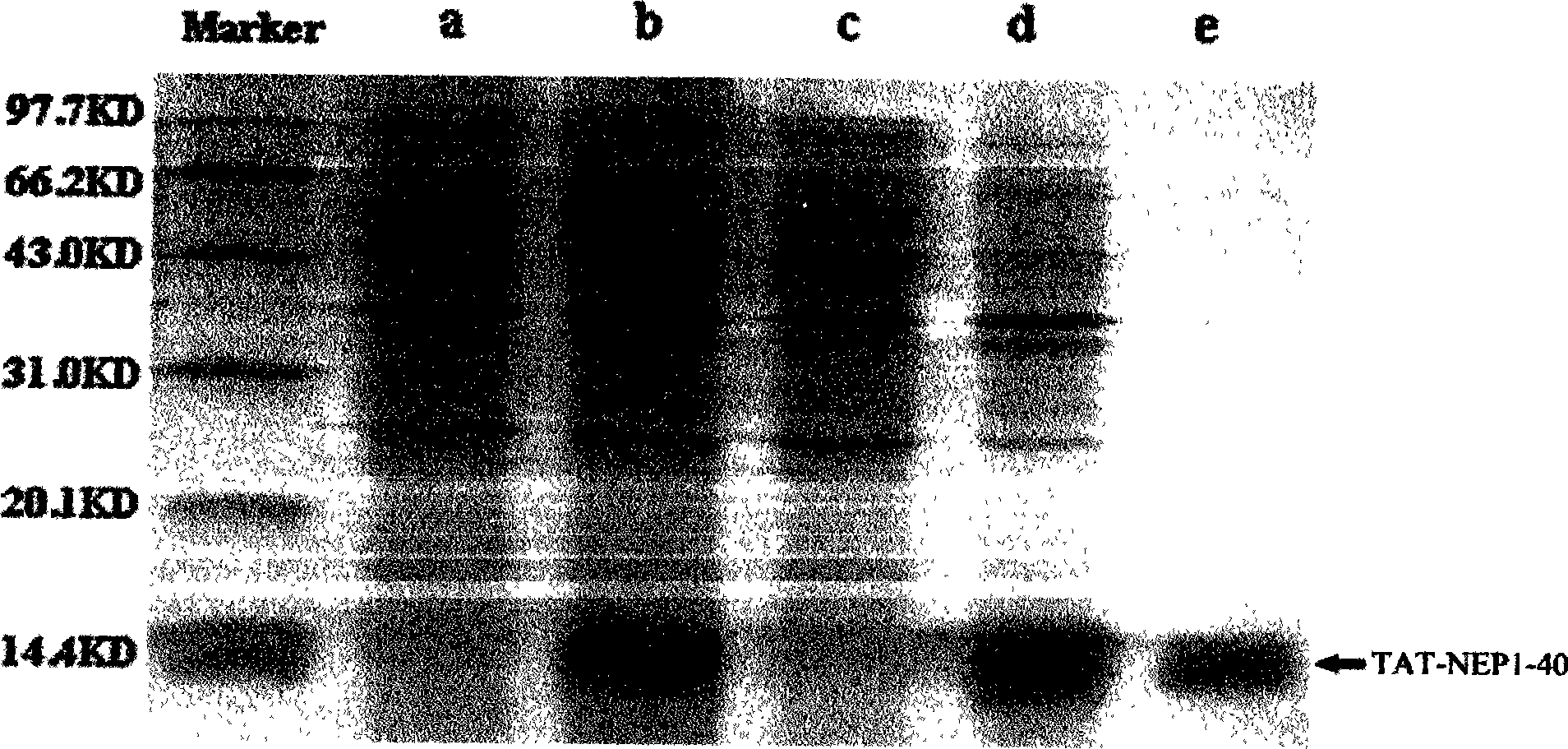TAT protein transduction modified NEP1-40 fusion protein and use thereof
A technology of protein transduction domain and fusion protein, which is applied in the fields of application, peptide/protein components, medical preparations containing active ingredients, etc., can solve problems affecting drug research and development, achieve high transduction efficiency and overcome limitations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Below in conjunction with specific example, further set forth the present invention. It should be understood that these examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention. The experimental method that does not indicate specific condition in the following examples, generally according to conventional conditions such as people such as Sambrook, molecular cloning: the condition described in the laboratory handbook (New York: Cold Spring Harbor Laboratory Press, 1989); Another example such as David Human, conditions described in Cellular Experiment Manual (New York: Cold Spring Harbor Laboratory Press, 1998).
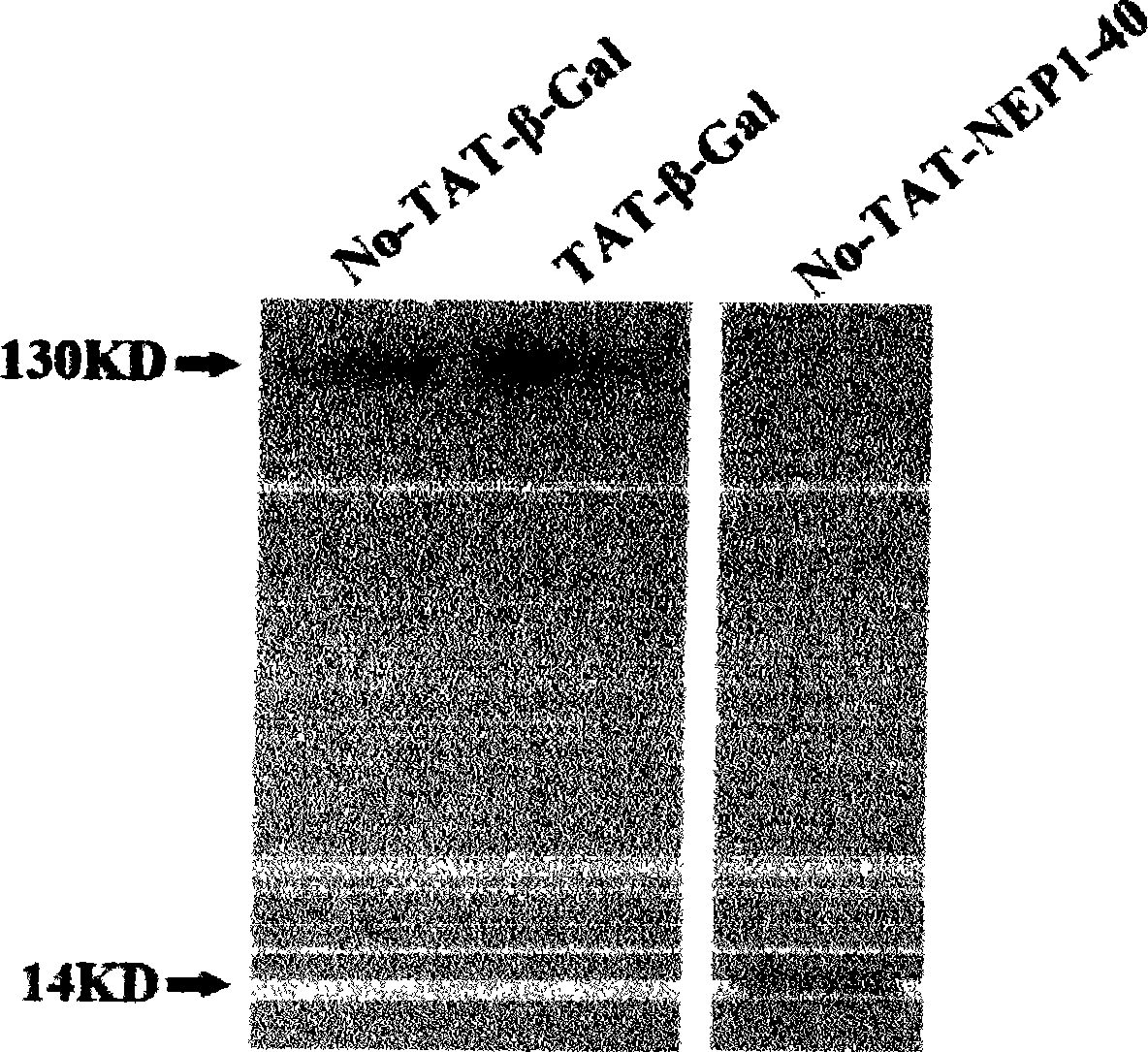
[0021] (1) Expression, purification and renaturation of TAT-NEP1-40 fusion protein
[0022] 1. Preparation of Escherichia coli competent cells (CaCl 2 Law):
[0023] (1) Pick a single colony of Escherichia coli DE3 in 5 ml of LB medium without Amp, and culture at 37°C overnight.
[0024] (2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com