TAT (Trans-activating factor) kringle domain-modified nenurogenin2 fusion protein, as well as preparation method thereof and application thereof
A fusion protein and structural domain technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, peptide/protein components, etc., to achieve the effect of low preparation cost and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
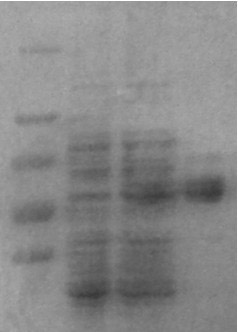
[0032] Embodiment 1: Preparation of the TAT-neurognin2 fusion protein of the present invention
[0033] 1. Obtain the pTAT-neurognin2 recombinant expression plasmid vector;
[0034] (1) Select the enzyme containing restriction endonuclease Nco I and xho I site, and the pTAT-HA expression vector containing the protein transduction domain PTD;
[0035] (2) Obtain the neurognin2 gene sequence from Genebank, and then optimize the gene sequence through prokaryotic expression;
[0036] (3) Synthesize the neurognin2 target gene fragment after prokaryotic expression optimization, and the two ends of the sequence contain Nco I and xho I restriction site, and loaded into the pUC57 vector to form pUC57-neurognin2 plasmid vector;
[0037] (4) use Nco I and xho I Two enzymes cut the pTAT-HA expression vector and the pUC57-neurognin2 target fragment plasmid vector into the same cohesive ends with double enzymes. Enzyme digestion reaction conditions: 37 ℃ 1h-16h, 65 ℃ ...
Embodiment 2
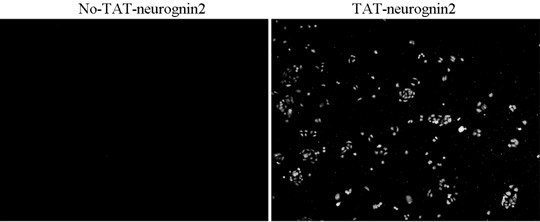
[0066] Example 2: Identification of the transduction function of the TAT-neurognin2 fusion protein of the present invention
[0067] 1. Identification of transmembrane transduction of TAT-neurognin2 fusion protein (such as image 3 shown);
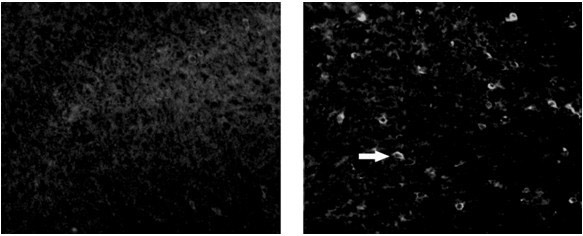
[0068] 2. Identification of TAT-neurognin2 fusion protein crossing the blood-brain barrier (such as Figure 4 shown);
[0069] In the present invention, the TAT-neurognin2 protein is injected intraperitoneally into the mouse, and after 6 hours, the brain of the anesthetized mouse is taken, and the frozen section is used to analyze the transduction effect of the TAT-neurognin2 fusion protein through the blood-brain barrier by immunofluorescence histochemistry. The results show that TAT- The neurognin2 fusion protein can pass through the blood-brain barrier and enter the brain parenchyma, while the brain tissues of mice injected with the No-TAT-neurognin2l fusion protein showed negative reactions, indicating that TAT can mediate protein ...
Embodiment 3
[0070] Example 3: The effect of the TAT-neurognin2 fusion protein of the present invention on inducing AS cells to differentiate into neurons in vitro
[0071] 1. Culture of mouse astrocytes
[0072] Take out the cerebral cortex of Kunming mice 7 days after birth, add it to the culture dish of D-Hanks solution, cut the tissue repeatedly with iris scissors until it becomes minced, suck the tissue into a 15ml centrifuge tube, add 0.125% trypsin (Sigma) 37 ℃ water bath for 20 minutes, add serum to the centrifuge tube to stop the digestion, blow gently with a round-tip dropper, let stand for 10 minutes, carefully draw the supernatant, add the supernatant to the pre-packed with poly-lysine (25μg / ml) Add DMEM / f12 containing 10% serum (Gibico) to the culture flask, change half of the medium on the third day, and then change the medium every three days, 12-15 days, when the cells are almost full, put the cells in the shaker Purify in the bed at 300 rpm for 15-19 hours, add new cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com