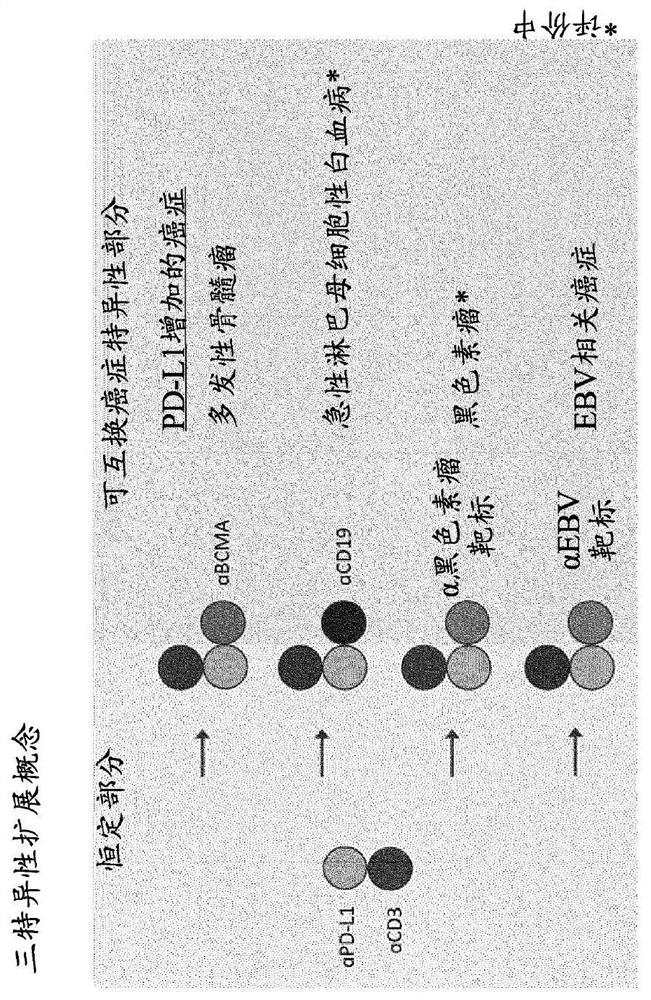
Trispecific antigen binding proteins
A protein-binding and specific technology, used in hybrid immunoglobulins, anti-animal/human immunoglobulins, immunoglobulins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
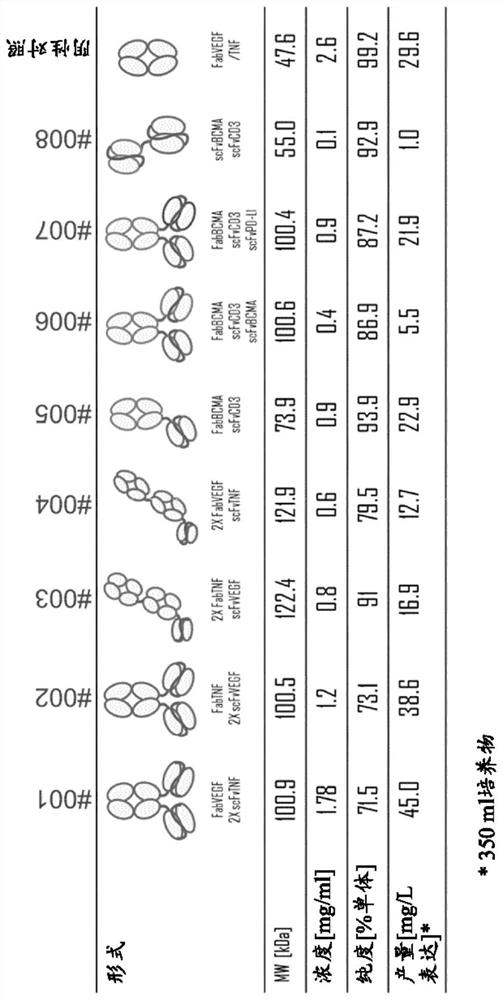
[0211] Example 1 - Design, Expression and Purification of Exemplary Trispecific Antigen Binding Proteins
[0212] background
[0213] A major challenge in the development of trispecific antigen-binding protein therapeutics is the selection of molecular forms from among structurally diverse alternatives that can support a variety of different biological and pharmacological properties while maintaining the desirable attributes of developability. Such attributes include high thermal stability, high solubility, low tendency to aggregate, low viscosity, chemical stability and high level of expression (gram per liter titer).
[0214] Production of trispecific antigen-binding proteins by co-expression of multiple (three) light and heavy chains in a single host cell can be highly challenging due to low yields of the desired trispecific antigen-binding protein, And it is difficult to remove closely related mismatched contaminants. In IgG-based trispecific antigen-binding proteins, ...
example 2- 3
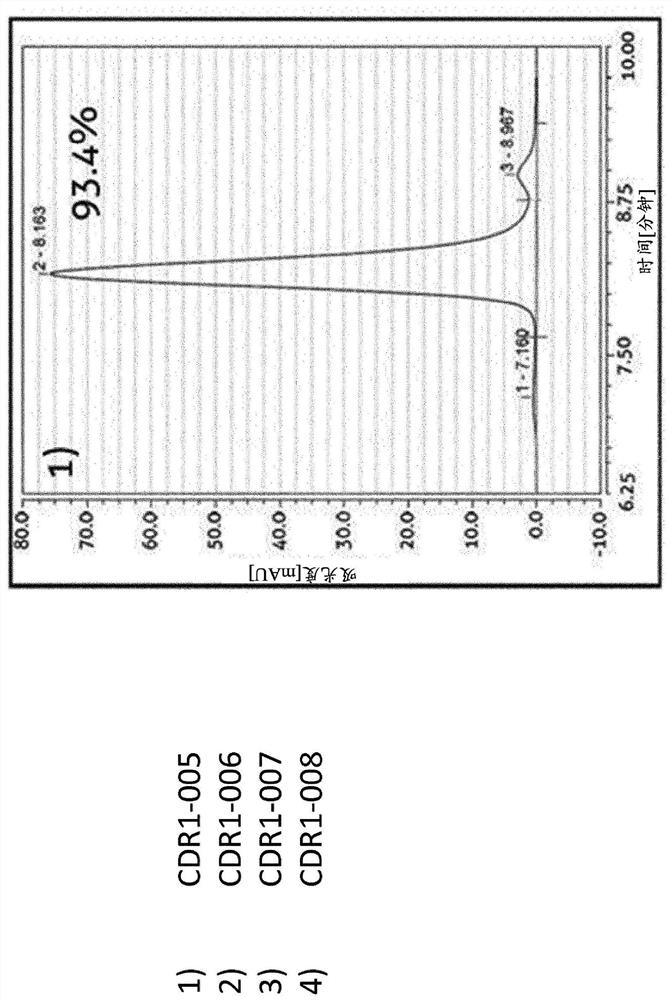
[0237] Example 2 - Ability of Trispecific Molecules and Bispecific Controls to Bind Their Targets
[0238] Binding ELISA assays were performed to determine whether the exemplary trispecific antigen binding proteins bound to their respective targets. The ability of the trispecific antibody CDR1-007 to bind its antigen was evaluated. Serial dilutions of CDR-007 to final concentrations ranging from 4 ng / mL to 10 μg / ml were tested in ELISA with human PD-L1 His-tag (nearside protein, #C315) recombinant human BCMA Fc chimera (produced in-house by transient expression in HEK293-6E cells) and the CD3εHis tag (proximity protein, #C578), each of which was coated on a 96-well plate. Trispecific antibodies were detected by goat anti-κ-LC antibody HRP (Thermo Fisher Scientific, #A18853). Figure 4A to Figure 4C Concentration-dependent binding of CDR1-007 was shown, confirming the ability of this trispecific antibody to bind three targets.
[0239] Additionally, the ability of trispecifi...
example 3-CD3
[0240] Example 3 - Ability of CD3 Binding Arm to Induce T Cell Proliferation
[0241] Antigen receptor molecules on human T lymphocytes are non-covalently associated with CD3 (T3) molecular complexes on the cell surface. Interference with this complex with anti-CD3 monoclonal antibodies may induce T cell activation, but this ability depends on certain properties, such as binding affinity, epitope, valency, antibody format, etc.
[0242] Linking different antigen-binding sites in fusion proteins to generate bispecific antibodies often exhibits reduced affinity for their target antigens compared to the parental antibody. Therefore, careful consideration should be given when evaluating the CD3-binding arms of T cell engagers to ensure functionality. One of the most common methods for assessing the ability of CD3 agonistic antibodies to activate T cells is to measure T cell proliferation after stimulation in vitro.
[0243] The CD3 binding arm design of the present invention was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com