Application of natural antibacterial peptide QHA in preparation of immunologic adjuvant
A natural antibacterial and immune adjuvant technology, applied in the direction of antibodies, antineoplastic drugs, antibody medical components, etc., can solve the problems of different antimicrobial peptide sequences and immunobiological effects that cannot be generalized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1QHA
[0030] (1) Synthesize the full sequence (shown in SEQ ID No.1) derived from the reptiles Qinghuanhai snake QHA with an automatic peptide synthesizer (433A, Applied Biosystems), and desalt and purify it by HPLC reverse-phase column chromatography.
[0031] (2) Molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF).
[0032] (3) The purity of the purified QHA was identified by high performance liquid chromatography (HPLC), the molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF), the isoelectric point was determined by isoelectric focusing electrophoresis, and the amino acid was determined by an automatic amino acid sequencer sequence structure.
[0033] The amino acid sequence of the natural antimicrobial peptide QHA of the present invention is shown in SEQ ID No.1. It consists of 30 amino acids...
Embodiment 2
[0034] Example 2QHA is used as an immune adjuvant to detect the impact of mouse antibody production
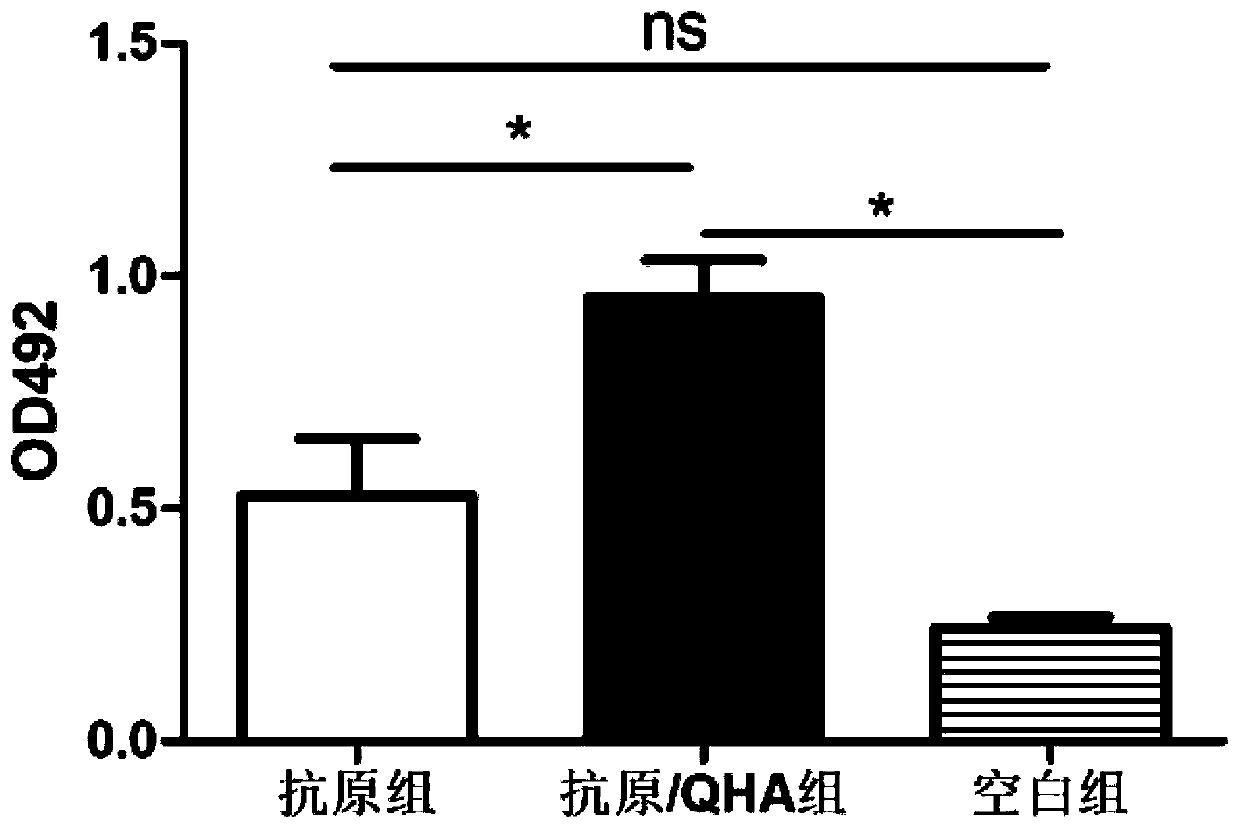
[0035] The experiments were divided into antigen group, antigen / QHA group and blank group (injected with the same volume of PBS), with 3 mice in each group.
[0036] The SARS-CoV-2 new coronavirus S1 protein antigen (provided by Suzhou Sige Bio) and the antimicrobial peptide QHA were dissolved in PBS to 1 mg / ml and 0.5 mg / ml, respectively, to obtain the antigen solution and QHA solution. In the antigen group, each mouse was immunized with 100 μl of antigen solution each time, and the immunization method was intraperitoneal immunization.
[0037] In addition, the above-prepared antigen solution was mixed with the QHA solution at a volume ratio of 1:1, and the resulting mixture was placed on a shaking table at 37°C and incubated for 35 minutes with shaking at 100 rpm / min to obtain an antigen / QHA mixture. In the antigen / QHA group, each mouse was immunized with 200 μl of antigen / ...
Embodiment 3
[0041] Embodiment 3 QHA combined with Freund's adjuvant as immune adjuvant for the detection of the impact of mouse antibody production
[0042] The experiments were divided into antigen / Freund's adjuvant group, antigen / QHA group and antigen / Freund's adjuvant / QHA group, with 3 mice in each group.
[0043] The SARS-CoV-2 new coronavirus S1 protein antigen (provided by Suzhou Sige Biological) and the antibacterial peptide QHA were dissolved in PBS to 1mg / ml and 0.5mg / ml to obtain the antigen solution and QHA solution. Then the antigen solution and Freund's adjuvant (volume ratio 1:1), antigen solution and QHA solution (volume ratio 1:1), antigen solution, Freund's adjuvant and QHA solution (volume ratio 1:1:1) were mixed , to obtain antigen / Freund's adjuvant mixed solution, antigen / QHA mixed solution and antigen / Freund's adjuvant / QHA mixed solution. When mixing, place the mixture on a vortex shaker for 35 minutes to vigorously shake to fully emulsify the Freund's adjuvant.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com