A fusion protein contraception vaccine with molecular adjuvant and its preparation
A fusion protein and adjuvant technology, which is applied in the direction of medical preparations containing active ingredients, drug combinations, and pharmaceutical formulas, can solve the problems of activating proto-oncogenes, destroying tumor suppressor genes, and causing cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
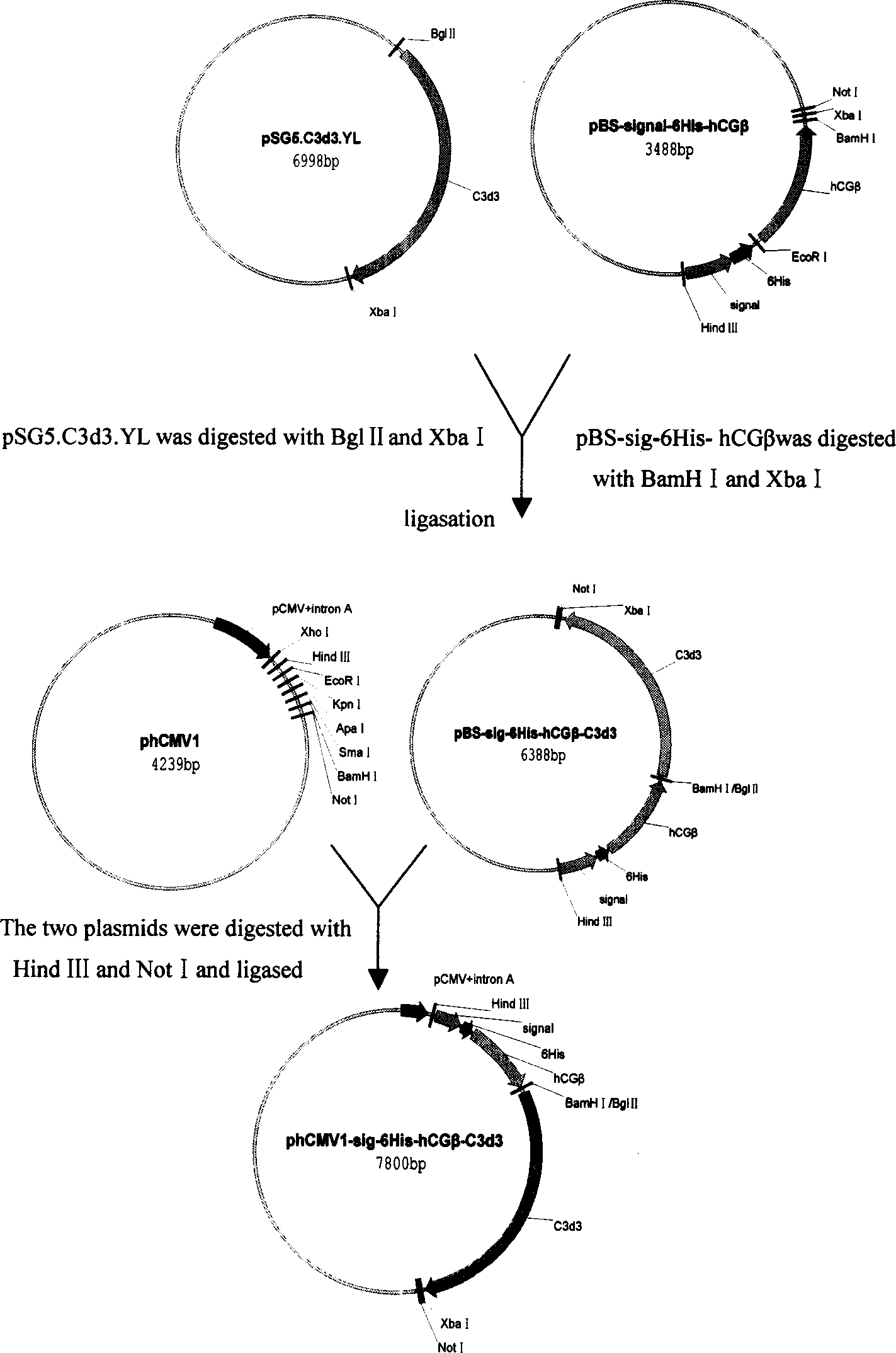
[0055] Example 1. Construction of secretory eukaryotic expression plasmids phCMV1-6His-hCGβ-C3d3 and phCMV1-6His-hCGβ.
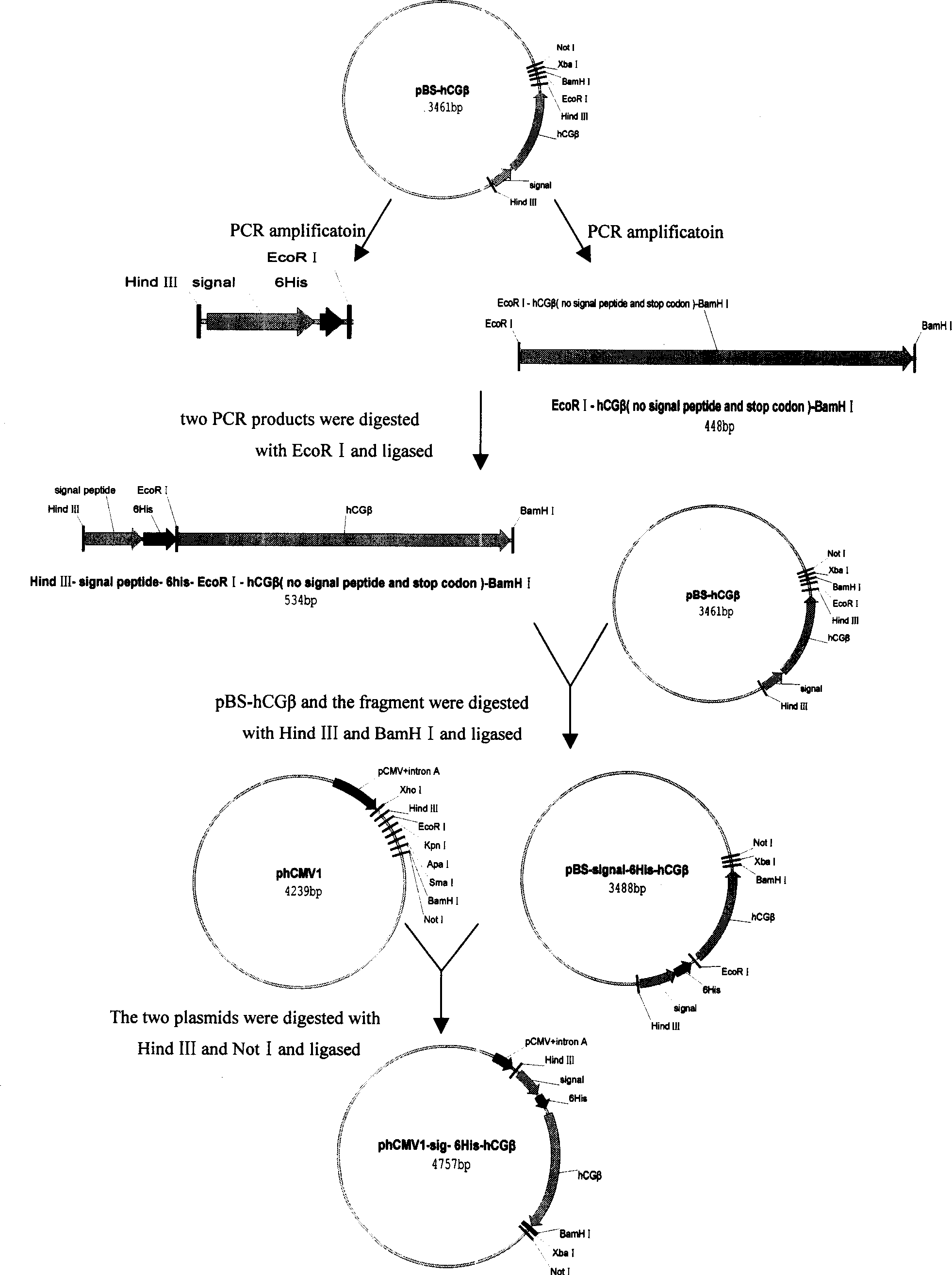
[0056] phCMV1-signal-6His-hCGβ construction strategy ( figure 2)
[0057] Using hCGβ with signal peptide as template, design the following 2 pairs of primers: 5'-CCAAGCTT ATG GAGATG TTC CAG GGG-3', 5'-CGGAATTC CCA ATG ATG ATG ATG ATG ATG TGC CCATGT CCC GCC CAT-3' and 5 '-CGGAATTC TCC AAG GAG CCGCTT CGG-3', 5'-CGGGATCC TTG TGG GAG GAT CGG-3'. Using pBS-hCGβ as a template, the Hind III-Signal-6His-EcoR I fragment was amplified with the first pair of primers. The EcoR I-hCGβ (no signal peptide and stop codon)-BamH I fragment was amplified with a second pair of primers. The Hind III-Signal-6His-EcoR I fragment was double-digested with Hind III and EcoR I, and the EcoR I-hCGβ (no signal peptide and stop codon)-BamH I fragment was double-digested with EcoR I and BamH I. Ligation obtained Hind III-Signal-6His-EcoR I-hCGβ (no signal peptide and stop codon)-BamH...
Embodiment 2
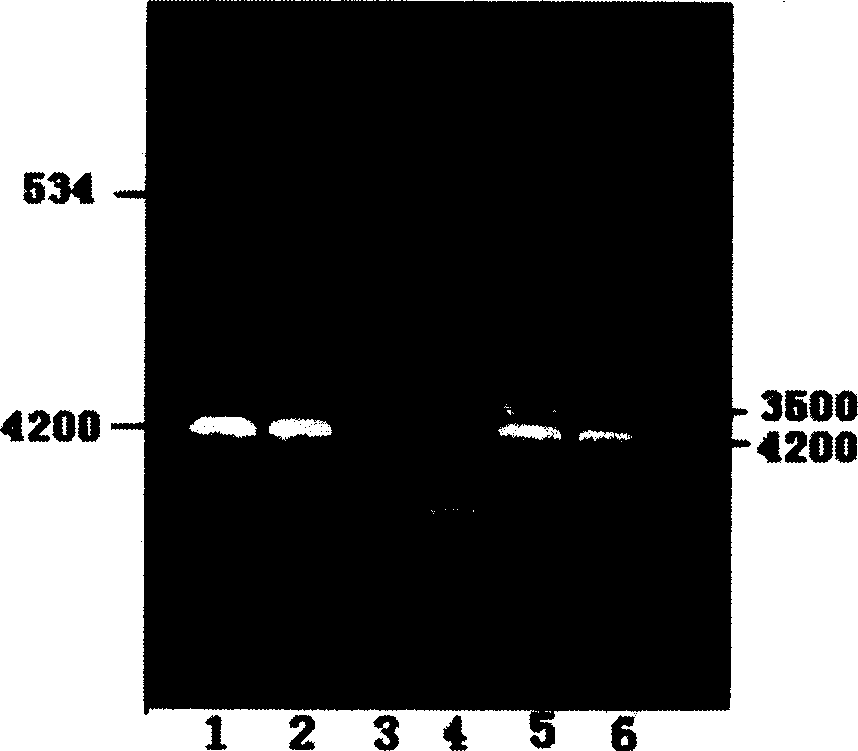
[0061] Example 2 Expression, identification and purification of hCGβ-C3d3 fusion protein and hCGβ protein.
[0062] Transfection of CHO cells with recombinant plasmids, selection of resistant clones and serum-free culture
[0063] When the CHO cells in the six-well plate were 95% confluent, use Lipofectamine2000 to mediate the transfection of CHO cells with phCMV1-6His-hCGβ-C3d3, phCMV1-6His-hCGβ, phCMV1, pcDNA3-hCGβ-C3d3 and pcDNA3-hCGβ respectively. And liposome dosage is 1μg:7μg. Positive clones were screened 24 hours after transfection with complete medium containing G418 (800ug / ml). The medium was changed every 3 days, and the selection was maintained for 8 days until the formation of drug-resistant cell clones. Select 10 positive clones of phCMV1-6His-hCGβ-C3d3 and phCMV1-6His-hCGβ respectively, maintain culture and amplification with culture medium containing G418 (300 μg / ml), and replace with CHO when 95% of the positive clones confluence into sheets cultured in ser...
Embodiment 3
[0072] Example 3. Animal Immunity and Immune Effects
[0073] Immunization method
[0074] 6-8 weeks old BALB / c mice (19-24g) and C57BL / 6 mice (20-25g) were divided into 3 groups, 5 mice in each group. They were immunized with hCGβ-C3d3 fusion protein, hCGβ alone and hCGβ plus Freund's adjuvant. The immunization was subcutaneously injected into the right back, and the immunization was done twice with an interval of 4 weeks. The immunization doses were: 50 pmol hCGβ-C3d3 fusion protein / 100 μl PBS, 50 pmol hCGβ / 100 μl PBS and 50 pmol hCGβ / 50 μl PBS+50 μl CFA formed complete emulsion. The hCGβ+CFA immunization group was immunized with hCGβ+IFA again.
[0075] Blood collection began 7 days after the first injection, once a week, a total of 4 times. Blood collection began 7 days after the second injection, once a week, a total of 3 times. Blood was collected from the retro-orbital venous plexus of mice, 100-150 μl each time, and 20-25 μl of serum was separated and stored at -2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com