Application of porphyrin pigment serving as immunologic adjuvant and vaccine
An immune adjuvant and pigment technology, applied in the field of immunology, can solve the problems of prolonged stimulation and limited enhancement effect, and achieve the effects of prolonged stimulation, promotion of absorption, and clear metabolic pathways in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The immunoenhancing effect of the combination of iron porphyrin, lecithin and iron porphyrin on the weak antigen bovine serum albumin was tested.
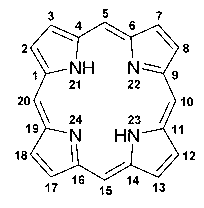
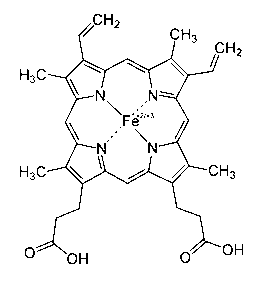
[0045] Iron porphyrin (FePP): also known as heme, the molecular formula is C 34 h 32 FeN 4 o 4 , the molecular mass is 616.49, the structure is as attached figure 2 Shown; Adopt the following method to prepare and obtain in the present embodiment, of course, also can adopt commercially available product.
[0046] (1) Collect animal or human anticoagulant blood, centrifuge at 4000 rpm, collect red blood cells, and wash red blood cells three times with 0.1M phosphate buffered saline (PBS, pH7.4).
[0047] (2) Add an equal volume of deionized water to the washed red blood cells, add 5 times the volume of chloroform after complete hemolysis and mix well, let it stand for about 10 minutes, and a cake-shaped hemoglobin precipitate will form on the upper layer of the 50mL centrifuge tube (the lower layer is clear chloroform so...
Embodiment 2
[0089] The immunoenhancing effect of the combination of cobalt porphyrin, lecithin and cobalt porphyrin on the weak antigen bovine serum albumin was tested.
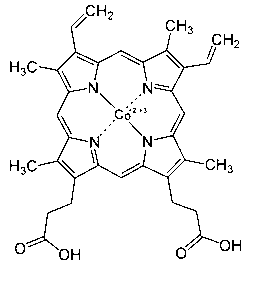
[0090] Cobalt porphyrin (CoPP): Molecular formula is C 34 h 32 CoN 4 o 4 , the molecular mass is 619.58, the structure is as attached image 3 As shown, the CoPP in this example was purchased from Sigma Company with a purity of ≥98%.
[0091] the solution
[0092] BSA powder (purchased from Sigma, purity ≥ 98%) was dissolved in 0.1 M phosphate buffer (PBS, pH 7.4) to prepare a 15 μM (1 mg / mL) BSA solution, which was stored in a refrigerator at 4 °C for later use.
[0093] the solution
[0094] First dissolve the CoPP powder with a small amount of 0.1M NaOH, then add PBS to make a 2mM (1.239mg / mL) CoPP solution, and store it in a 4°C refrigerator for later use.
[0095] the solution
[0096] The 15 μM BSA solution was mixed with an equal volume of 2 mMCoPP solution, so that the final concentrations of BSA and CoPP w...
Embodiment 3
[0118] To test the immune effect of hepatitis B vaccine containing iron porphyrin pigment on BALB / c mice.
[0119] Iron porphyrin (FePP)
[0120] Prepared by the same method as in Example 1, the purity is greater than or equal to 95%. Of course, commercially available products can also be used.
[0121] (HBsAg) solution
[0122] The product of Shanghai Yemin Biotechnology Co., Ltd., the purity is ≥95%, and the concentration is 2.5mg / mL.
[0123] FePP solution
[0124] First dissolve the FePP powder with a small amount of 0.1M NaOH, then add PBS to make a 2mM (1.233mg / mL) FePP solution, and store it in a 4°C refrigerator for later use.
[0125] the solution
[0126] An appropriate amount of HBsAg stock solution was diluted 5 times and mixed with an equal volume of 2mM FePP solution. The final concentrations of HBsAg and FePP were 0.25mg / mL and 1mM, respectively, and stored in a 4°C refrigerator for later use.
[0127] the solution
[0128] (1) Add 30mg of lecithin (PC) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com