Streptococcus suis B cell dominant epitope tandem vaccine and preparation method thereof
A dominant epitope, Streptococcus suis technology, applied in chemical instruments and methods, antibacterial drugs, pharmaceutical formulations, etc., can solve problems such as unanalyzed design, and achieve convenient and safe preparation process, good stability, and high safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Epitope Screening
[0057] (1) Protein primary structure analysis
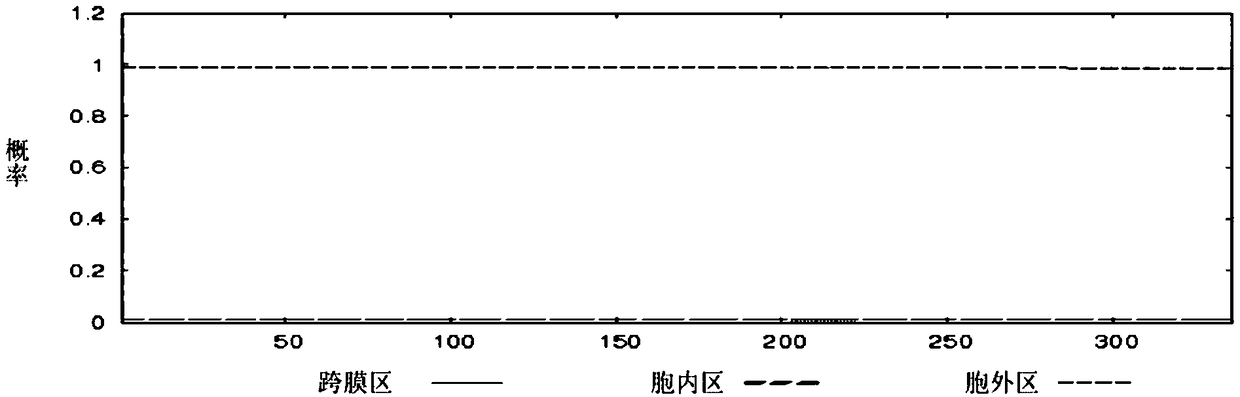
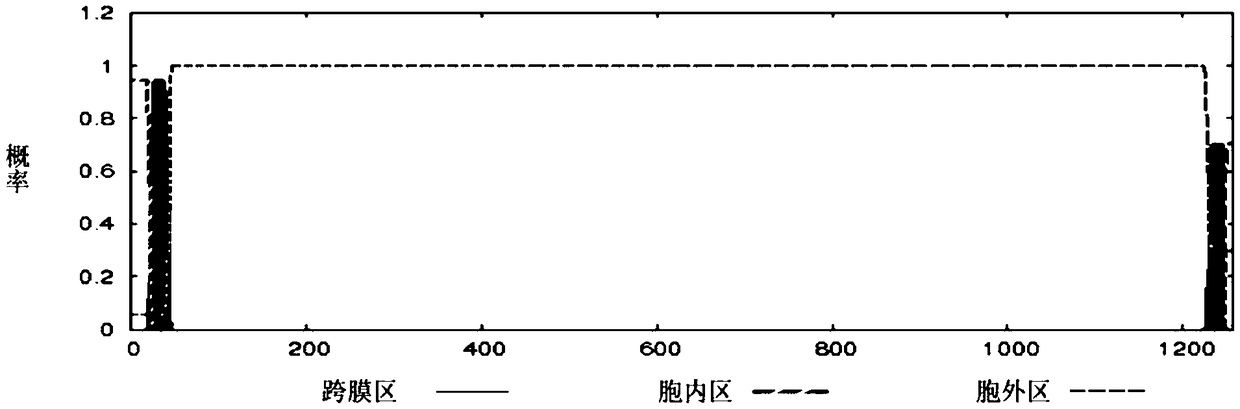
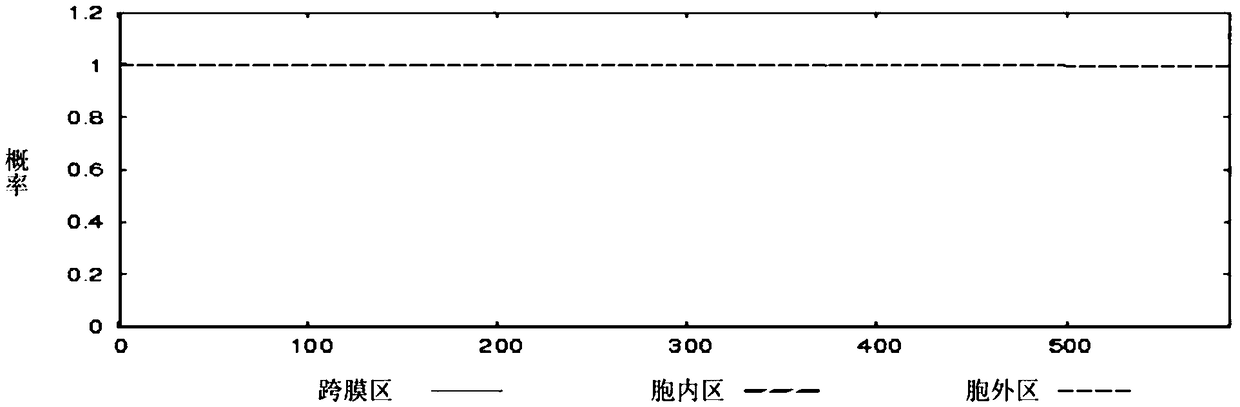
[0058] According to the GAPDH, MRP, and DLDH protein amino acid sequences of Streptococcus suis ZY05719 strain published by NCBI (accession numbers are AKG39592.1, AKG40097.1, and AKG41058.1), the online TMHMM tool (http: / / www.cbs.dtu .dk / services / TMHMM / ) to analyze the transmembrane region of the protein, and use the online SignalP tool http: / / www.cbs.dtu.dk / services / SignalP / to analyze the signal peptide region of the protein.
[0059] The results predicted by TMHMM tool analysis are: GAPDH protein has no transmembrane region, and its 1-336 amino acid sequences are all extracellular fragments (such as figure 1 shown); MRP protein has a transmembrane region located at amino acids 23-45, such as figure 2 As shown, the N-terminus of the protein is inside the cell, and the 46-1256 amino acid is outside the cell; the 1-586 amino acid of the DLDH protein is an extracellular fragment, and there is no tran...
Embodiment 2
[0069] Tandem epitope vaccine design and plasmid construction
[0070] According to the predominant B cell antigen epitopes of GAPDH, MRP, and DLDH proteins predicted in Example 1, all epitopes were combined and spliced in sequence according to the order of GAPDH-MRP-DLDH, and GGGG flexible fragments were used as linker amino acids between polypeptides to reduce Interactions between epitopes. The amino acid sequence of the GAPDH-MRP-DLDH tandem epitope protein is shown in SEQ ID NO: 2, and the amino acid number of the recombinant protein is 291aa. According to the original reference sequence of GAPDH (Genbank accession number: ZY05719_00895), MRP (Genbank accession number: ZY05719_03650), DLDH (Genbank accession number: ZY05719_08710), the above tandem sequences were codon optimized and BamHI and XhoI were introduced at the N-terminus and C-terminus respectively Restriction sites, the nucleotide sequence of the GAPDH-MRP-DLDH tandem epitope protein finally obtained is 888bp...
Embodiment 3
[0072] Prokaryotic expression and purification of tandem epitope protein GMD
[0073] (1) Prokaryotic expression of epitope protein. The recombinant plasmid pET-28a(+)-GMD constructed in Example 2 was heat-shocked at 42°C to transform E. coli competent cells BL21 (purchased from Beijing Quanshijin Biotechnology Co., Ltd.), and the recovered competent bacteria were subjected to 5000 rpm After centrifugation for 5 minutes per minute, the cells were resuspended in 100 μL of LB liquid medium, and the resuspended cells were spread on LB plates with kanamycin and cultured overnight in a 37°C incubator. Single clones on the plate were selected with a sterile pipette tip, placed in 1 mL of LB liquid medium containing kanamycin (50 μg / mL), and cultured on a constant temperature shaker at 37°C at 220 rpm for 4 hours. Colony PCR method was used for cloning identification. The primers used in PCR were: upstream primer GMD-F: 5'-ACGGGATCCGAACCGGGTAATATT-3', downstream primer GMD-R: 5'-ACG...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com