Mouse-typhus salmonella gene-deletion mutant strain without containing resistance marks, vaccine and application thereof
A gene deletion vaccine, Salmonella technology, applied in the field of animal bacterial genetic engineering, can solve the problems of not being able to be used as a vaccine strain, not meeting the biosafety requirements, etc., and meeting the vaccine biosafety requirements, good immune protection, and good safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Salmonella typhimurium SL1344 cya Construction of gene deletion strain SL13441
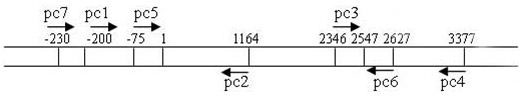
[0038] 1, cya Related primer design
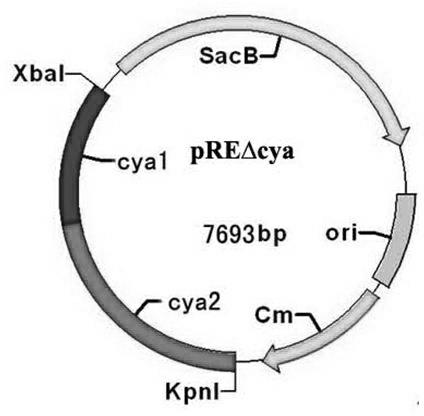
[0039] Refer to http: / / www.ncbi.nlm.nih.gov / registered Salmonella typhimurium LT2 strain cya Gene sequence (GenBank No: AE006468.1) design 7 pieces cya Related primers (see figure 1 , Table 1), for Salmonella typhimurium parent strain SL1344 (gifted by Nanjing Agricultural University) and gene deletion strain ?cya Construction and characterization of SL1344. All primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd.
[0040] Table 1 PCR primers
[0041]
[0042] 2. Salmonella typhimurium SL1344 cya Gene Upstream and Downstream Fragments cya 1 (upper arm) with cya Cloning of 2 (lower arm)
[0043] Put Salmonella typhimurium SL1344 on LB solid medium (10g tryptone, 5g yeast extract, 10g sodium chloride, 12g agar powder, ultrapure water to 1000mL, autoclave at 121°C for 20min) in a zigzag shape Line, cultured...
Embodiment 2
[0049] Example 2 Salmonella typhimurium cya Biological characteristics of gene deletion strain SL13441
[0050] 1. Phenotype identification of gene deletion strain SL13441
[0051] The serotype of the deletion strain SL13441 was identified according to the instructions of Salmonella single-factor serum (purchased from Zhejiang Ningbo Tianrun Bio-Pharmaceutical Co., Ltd.). The gene deletion strain SL13441 (experiment number: deletion strain ?cya SL1344) and the parental strain SL1344 were inoculated on MacConkey agar plates with and without 1% maltose, and then transferred to glucose, maltose, lactose, sucrose, arabinose, xylose, mannitol and other carbon sources and H 2 S and other biochemical identification tubes (purchased from Hangzhou Tianhe Microbial Reagent Co., Ltd.) were used to study their biochemical characteristics. The results showed that the serotype of the gene deletion strain SL13441 was consistent with that of the parental strain SL1344, which was still 1,...
Embodiment 3
[0059] Example 3 Preparation of Salmonella typhimurium cya gene deletion strain SL13441 vaccine
[0060] The gene deletion strain SL13441 (experiment number: deletion strain?cyaSL1344) was streaked and cultured on LB solid medium for 24 hours, the culture was scraped and washed with sterile saline, centrifuged at 10000r / min for 5min, the supernatant was discarded, and an appropriate amount of Sterilize the suspension in saline. Centrifuge at 10000r / min for 5min, wash 1-2 times. After suspending the bacteria with sterilized normal saline, serially 10-fold dilution was used to determine the number of viable bacteria by plate counting method, and finally the appropriate bacterial concentration was adjusted with sterilized normal saline according to the needs of the test to obtain a spare vaccine for the test.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com