Haemophilus parasuis attenuated salmonella vaccine
A technology of Haemophilus suis and Salmonella, applied in bacteria, antibacterial drugs, bacterial antigen components, etc., can solve the problems of economic losses in the pig industry, antibiotics threatening human health and public health safety, etc., and achieve good immune protection, The effect of good humoral immunity and broad market application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
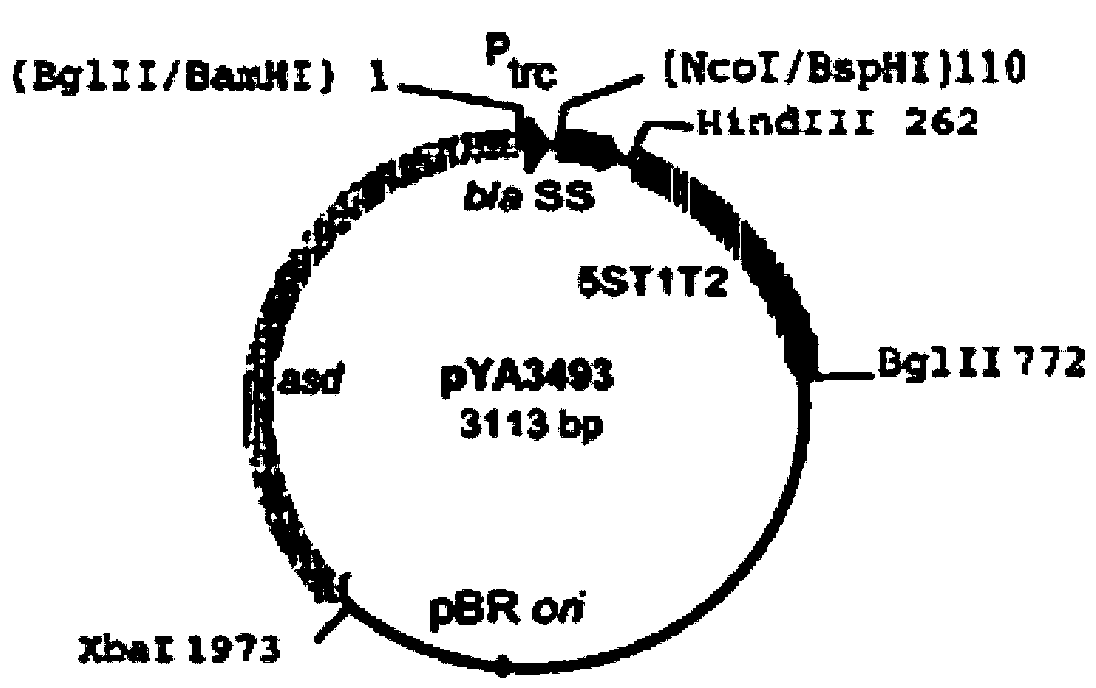
[0042] Example 1: Cloning of Haemophilus parasuis outer membrane antigen gene hps06257 and construction of recombinant plasmid pYA-06257
[0043] 1. Main experimental materials
[0044] Escherichia coli χ6097 (ara Δ (lac-pro)rpslΔasdA4Δ[xhf2::Tn10]thiΦ80d / lacZΔM15) was kindly provided by Dr.Roy CurtissIII, University of Washington, USA. CaCl 2 , Glycerin and other reagents are products of Shanghai Sinopharm Chemical Reagent Co., Ltd. PCR-related reagents such as Taq enzyme, endonucleases such as EcoR I and BamH I and related Buffers, T4 ligase and corresponding Buffers, DH5α competent cells, etc. are all products of Treasure Bioengineering (Dalian) Co., Ltd. NAD (nicotinamide adenine dinucleotide) and DAB chromogenic diagnostic kits are all products of Sigma Company, and newborn bovine serum is a product of Hangzhou Sijiqing Biological Products Co., Ltd. TSB, TSA for Difco TM product. Bacterial Genomic DNA Extraction Kit was purchased from Tianjin Tiangen Biochemical Tec...
Embodiment 2
[0063] Example 2 Construction of Salmonella choleraesuis asd gene deletion mutant strain asd-C500
[0064] 1. Main experimental materials
[0065] Attenuated commercial vaccine strain C500 of Salmonella choleraesuis was purchased from China Veterinary Drug Control Institute. pBluescriptSK(+) carrier plasmid (see attached for the physical map of the plasmid Figure 4 ) was purchased from Stratagene, USA. Suicide plasmid pRE112 (see attached Figure 5 ), Escherichia coli χ7213 strain was kindly donated by Professor Dr. Roy Curtiss III, University of Washington, USA.
[0066] PCR-related reagents such as Taq enzyme, endonucleases such as Xba I and BamHI, related Buffer, T4 ligase and Buffer, DH5a competent cells, etc. are all products of Treasure Bioengineering (Dalian) Co., Ltd. Bacterial Genomic DNA Extraction Kit was purchased from Tianjin Tiangen Biochemical Technology (Beijing) Co., Ltd. UNIQ-10 Column DNA Gel Recovery Kit was purchased from Shanghai Sangon Bioengineering...
Embodiment 3
[0079] Embodiment 3: recombinant Salmonella choleraesuis asd - C500 / pYA-06257 strain construction
[0080] 1. Salmonella choleraesuis asd - Preparation of C500 Competent Cells
[0081] Removal of Salmonella choleraesuis asd from -80°C freezer - C500 freeze-dried powder, directly dipped with sterile platinum wire and streaked on the surface of LB plates containing 50 μg / mL DAP, cultured upside down at 37°C for 16-18 hours; pick a single colony and inoculate into 4 mL LB containing 50 μg / mL DAP In the culture medium, cultivate overnight at 37°C and 180r / min; transfer the culture to 50mL LB culture medium (containing 50μg / mL DAP) at a volume ratio of 1:100, and culture with shaking at 37°C and 230r / min for 2-3h; take out 100-200 μL culture to detect OD 600 , when OD 600 When the temperature is around 0.8, cool the bacteria solution to 0°C in an ice bath; then centrifuge at 5000r / min for 10 minutes at 4°C to collect the bacteria, discard the supernatant; wash the bacteria ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com