Avian Escherichia coli vaccine strain
An avian Escherichia coli and Escherichia coli technology, applied in vaccines, veterinary vaccines, bacteria, etc., can solve the problem of unsatisfactory use of Escherichia coli inactivated vaccines, poor immune effect of aluminum hydroxide glue vaccines, and large side effects of oil-emulsion vaccines. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Separation and purification of bacterial strains and analysis of their physical and chemical properties
[0017] 1) In June 2018, a serious Escherichia coli outbreak occurred in a chicken farm in Shandong. The heart, liver, spleen, etc. of dead chickens were collected, sealed in sampling bags, and brought back to the laboratory and placed in a 4°C ice box. Save for later.
[0018] 2) Grind the collected chicken organs and apply them on the chromogenic medium to display different colored strains. Every 3 bacterial strains showing different colors are inoculated into LB liquid medium as a group for expanded culture, and the thalline is collected by centrifugation at 10000rpm. Then the bacterium was resuspended with 0.5% sterile PBS, and the bacterial concentration of the bacterial strain was determined as 1×10 by turbidimetric method. 7 CFU / ml.
[0019] 3) Strong toxicity separation experiment:
[0020] The isolated bacterial strains were respectively cultur...
Embodiment 2
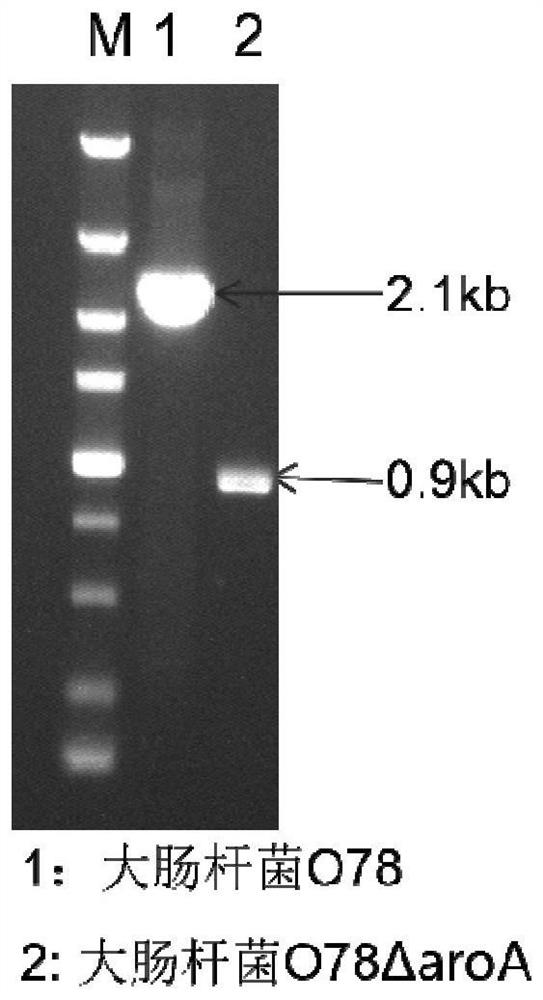
[0032] Example 2: Knockout and detection of the aroA gene of avian Escherichia coli YBO78
[0033] 1) According to the aroA gene sequence of avian Escherichia coli O78 on NCBI GenBank, design sequencing primers (Table 3), amplify the aroA gene of clinical isolate YBO78 by PCR, and sequence and identify it.
[0034] Table 3: Sequence Information Table of Sequencing Primers
[0035] Upstream sequencing primers Downstream sequencing primers O78-seq-F1 O78-seq-R1 O78-seq-F2 O78-seq-R2 O78-seq-F3 O78-seq-R3
[0036] 2) Design primers for aroA gene knockout according to the sequencing results (Table 4).
[0037] Table 4: Primer sequence list for aroA gene knockout
[0038]
[0039] 3) Construct the recombinant plasmid pSC101-BAD-gbaA-cm, and electroporate into Escherichia coli YBO78 bacteria after the sequencing is correct.
[0040] 4) For arabinose induction, the kanamycin gene cassette loxp-kan-loxp was electrotransferred into Escherichia...
Embodiment 4
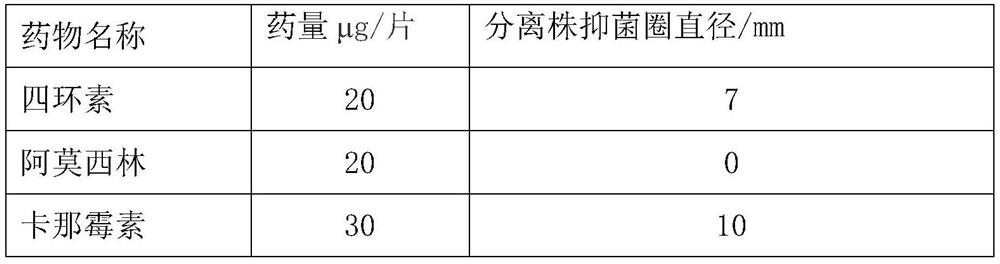
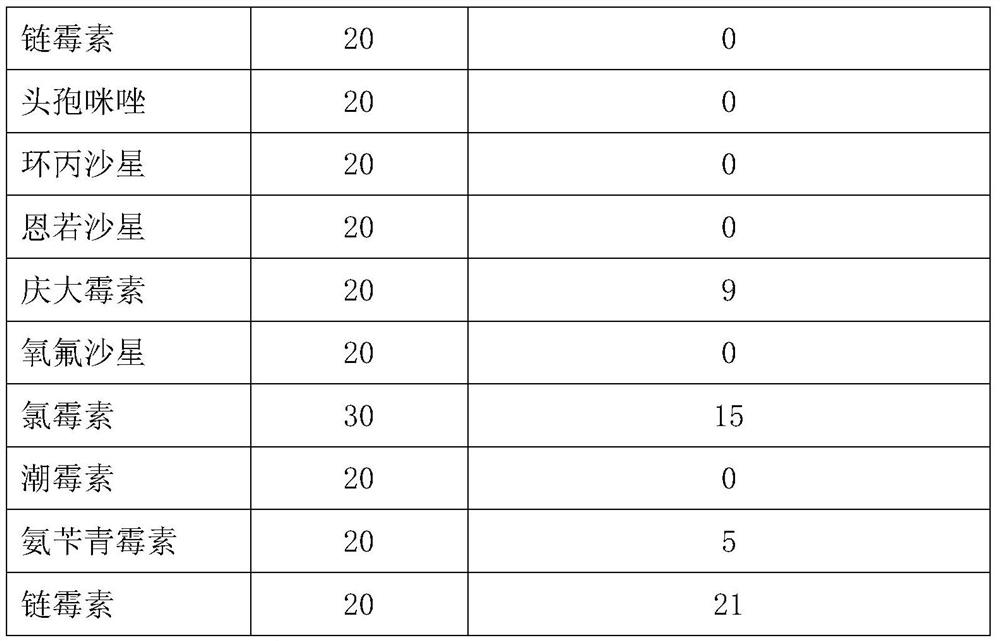
[0046] Embodiment 4: Biosafety evaluation of YBO78-1 strain
[0047] First, inoculate the Escherichia coli YBO78-1 strain on the LB solid plate medium without anti-antibody, culture overnight at 37°C, then pick a single colony and inoculate it into 50ml of anti-LB liquid medium, place it in a small shaker, 37 After culturing overnight at 200 rpm at ℃, centrifuge at 10,000 rpm for 1 min to collect the precipitate, resuspend the precipitate with sterile PBS, count the viable bacteria of the bacterial suspension, and prepare for subsequent animal injection infection experiments.
[0048] Twenty-five 30-day-old SPF chickens were selected and randomly divided into 5 groups with 5 chickens in each group. Dilute the YBO78-1 bacterial solution to 1.0×10 8 CFU / ml, 2.0×10 8 CFU / ml, 4.0×10 8 CFU / ml, 8.0×10 8 Four different gradients of CFU / ml were used to inoculate 5 experimental SPF 1.0ml each, and the other group was injected with 1.0ml isolated wild YBO78 strain as a control.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com