Methods of making vanillin via the microbial fermentation of ferulic acid from eugenol using a plant dehydrogenase.
A technology of ferulic acid and vanillin, applied in the field of identification of natural vanilla products, can solve problems such as product errors and wrong evaluations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
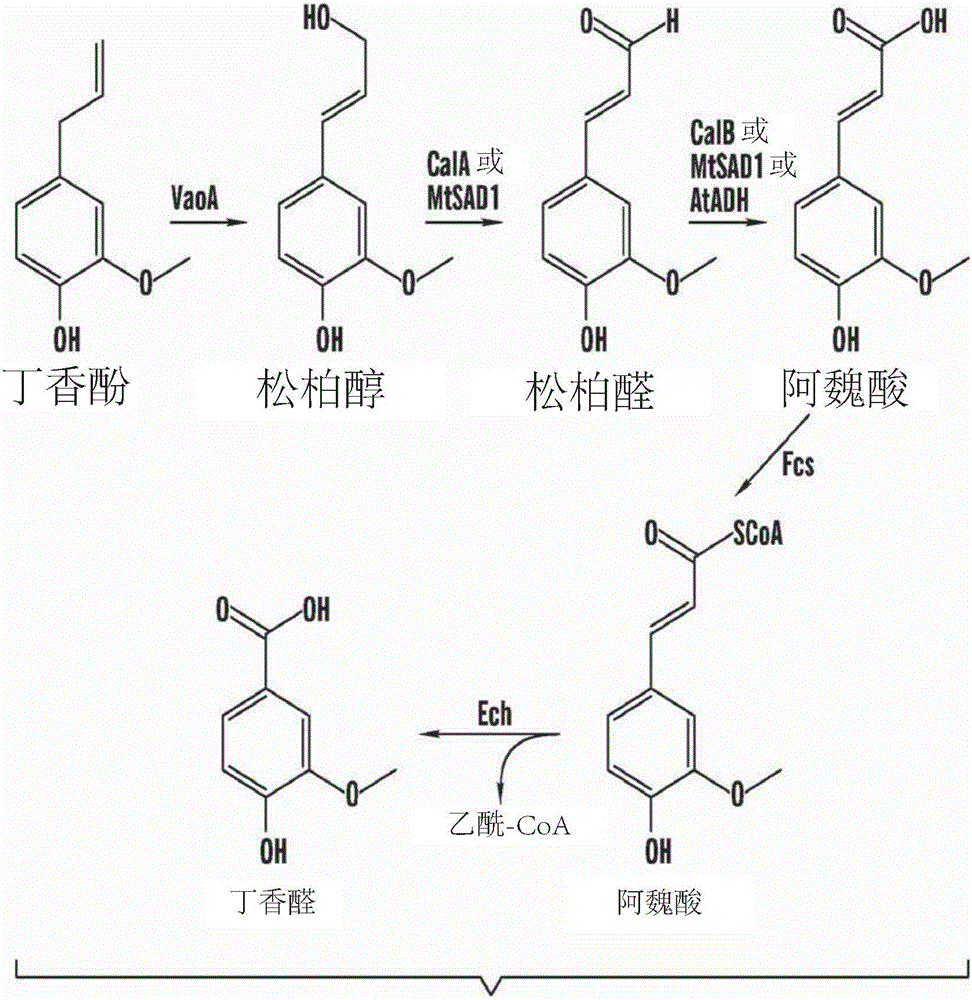
[0303] Example 1. Generation of ferulic acid from eugenol in a 25ml (0.025L) shake flask
[0304] Short-chain alcohol dehydrogenase (MtSAD1) and aldehyde dehydrogenase (AtADH) were cloned from Medicago truncatula and Arabidopsis thaliana, respectively. MtSAD1 and AtADH were cloned from Medicago truncatula (ecotype A17) and Arabidopsis thaliana (ecotype Columbia-0). Total plant RNA was extracted using Trizol Plus RNA Purification Kit (Invitrogen Inc.). Using Im Prom-II from Promega Inc. TM The reverse transcription system was used for cDNA synthesis according to the manufacturer's manual. Using the primers listed in Table 2, the gene was amplified from the synthesized cDNA using New England Biolab's Phusion PCR kit.
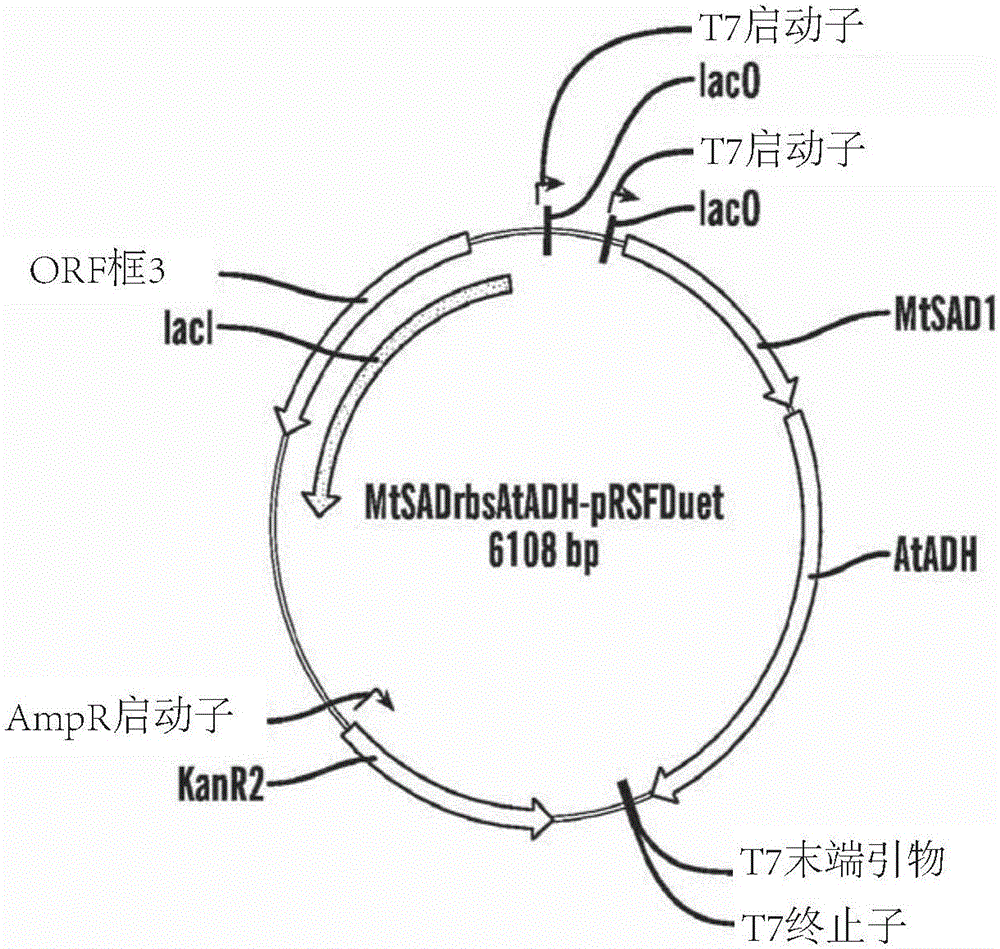
[0305] After digesting the PCR product with corresponding restriction enzymes, MtSAD1 was inserted into the Nde I and Xho I cloning sites of the pRSFDuet-1 vector. Insert AtADH into the Bgl II and Xho I cloning sites of pCDFDuet-1. After sequence confirmation...
Embodiment 2A
[0342] Example 2A. Ferulic acid production using eugenol in a 30 L volume in a 50 L fermenter
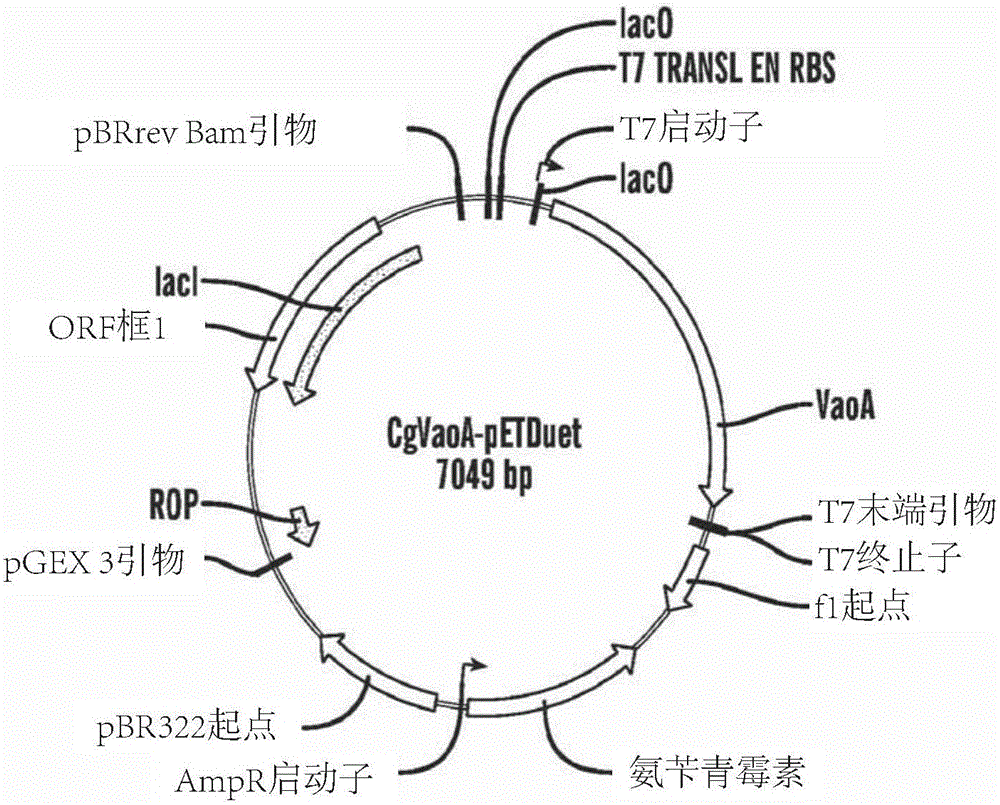
[0343] A 1 ml glycerol stock solution of E. coli cells containing CgVaoA-pETDuet and MtSADrbsAtADH-pRSFDuet was inoculated into 1 L of LB medium containing 100 mg / L ampicillin and 30 mg / L kanamycin and seeded at 37 Cultivate overnight at °C, and then transfer to 30 L LB medium containing 100 mg / L ampicillin and 30 mg / L kanamycin in a 50 L fermenter. The initial temperature was set at 37 °C, stirring at 300 rpm, the dissolved oxygen (DO) was kept above 30%, the air was 0.6 vvm, and the pH was not controlled at the beginning. After 1-1.5 hours, the temperature was lowered to 30 °C and 2 L of 22.5 g / L lactose was added to bring the final concentration to 15 g / L to start the induction. At this point the volume is about 33-34 L. Keep the air at 0.6vvm, increase the agitation to 350rpm, and keep the DO above 15%. After 14-16 hours of fermentation, the substrate eugenol was added, the t...
Embodiment 2B
[0351] Example 2B Biotransformation of Ferulic Acid to Vanillin
[0352] Purified ferulic acid (FA) converted from eugenol using our VaoA-MtSAD1-AtADH system was used as substrate for vanillin production. FA was converted to natural vanillin (NV) using Amycobacterium sp. strain (Zhp06) and the method described in US 2013 / 0115667A1 and CN102321563B1. Applicant's results showed that a yield of 13.4 g / L vanillin was obtained from 21.6 g / LFA, corresponding to a molar yield of vanillin of 79.2% ( Figure 8 ). During fermentation, the accumulation of vanillin was roughly linear. However, Applicants observed a rapid decrease in FA in the first 24 hours, suggesting that S. chlorosporium converts FA to a different intermediate before FA is converted to vanillin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com