Preparation method of recombinant uricase
A uricase and recombinant protein technology, applied in the fields of genetic engineering and protein biochemistry, can solve the problem of not solving the method of efficient fusion expression of uricase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
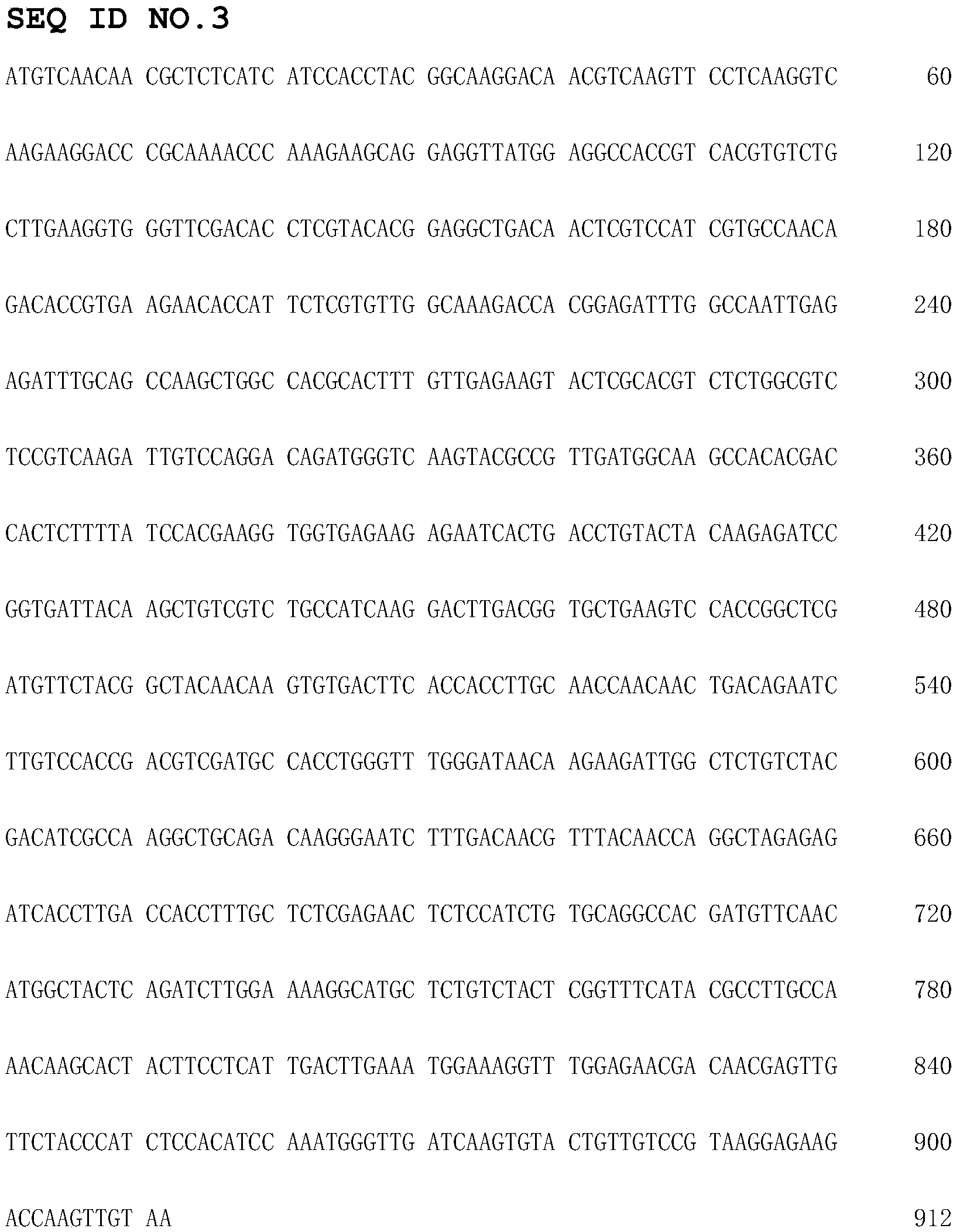
[0119] Cloning and expression of embodiment 1 Candida utilis uricase
[0120] 1. Extraction of Candida utilis genomic DNA: After resuscitating the freeze-dried Candida utilis (2.0120) strain according to the instruction manual, inoculate it into YPD culture (yeast extract 5g, peptone 10g, glucose 10g, add water to 500ml, Adjust the pH to 6.0) and cultivate in shake flasks at 30°C. After two consecutive generations of culture, Candida utilis can be seen to grow normally. The cells were collected by centrifugation, and the genomic DNA was extracted with a yeast genomic DNA extraction kit.
[0121] 2. Amplification of the target gene: According to the gene sequence of Candida utilis uricase in GenBank, a pair of primers were designed and synthesized (synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.), and NdeI and HindIII restriction sites were introduced into the primers.
[0122] P1: 5'-ggA ATT CCA TAT gTC AAC AAC gCT CTC ATC-3'
[0123] P2: 5'-CCC AA...
Embodiment 2
[0128] Purification and identification of embodiment 2 recombinant uricase
[0129] Purification of the target protein: centrifuge the induced bacterial solution at 5000r / min and 4°C for 20min, collect the bacterial cells, wash them twice with PBS (pH7.4), resuspend the bacterial cells with PBS, and ultrasonically disrupt the bacterial cells ( The whole process was carried out in an ice bath), centrifuged at 12000r / min, 4°C for 45min, and the supernatant was collected; the supernatant was subjected to ammonium sulfate fractional precipitation, and the recombinant uricase was mainly contained in the 40%-60% ammonium sulfate saturation fraction Protein, the precipitate of this fraction was collected, dissolved in 50mmol / L glycine buffer (pH10.0), desalted with Sephadex G25, and then purified with Resource Q anion exchange column ( Figure 10 ), collected the eluate containing recombinant uricase, and carried out molecular sieve purification with a Superdex 75 column ( Figure 1...
Embodiment 3
[0132] The activity measurement of embodiment 3 recombinant uricase
[0133] Activity analysis: The method reported by Legoux et al. (1992) was used. The amount of enzyme required to oxidize 1 μmol of uric acid to allantoin per minute is 1 International Unit (IU) of enzyme activity. In a 5ml reaction system, add 3ml triethanolamine buffer solution (pH8.9), 1.5ml 0.2μmol / L uric acid stock solution, 1μg (10μl) purified uricase, react at 30°C for 5min, then add 0.5ml 20% KOH was used to terminate the reaction, and the concentration of uric acid was measured with a detection wavelength of 292nm.
[0134] The activity of the enzyme was calculated according to the standard curve, and there was no difference in the activity of the recombinant uricase expressed from the wild-type Candida utilis uricase gene and the optimized sequence, and the specific activities were 36.1U / mg and 37.9U / mg, respectively.
[0135] The above results are summarized in Table 1.
[0136] Table 1 Activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com