Recombinant amidase Dt-Ami 2, encoding gene, vector, engineering strain and applications of recombinant amidase Dt-Ami 2 and engineering strain
A dt-ami2, encoding gene technology, applied in genetic engineering, recombinant DNA technology, applications, etc., can solve the problems of restriction amidase application and limited amidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
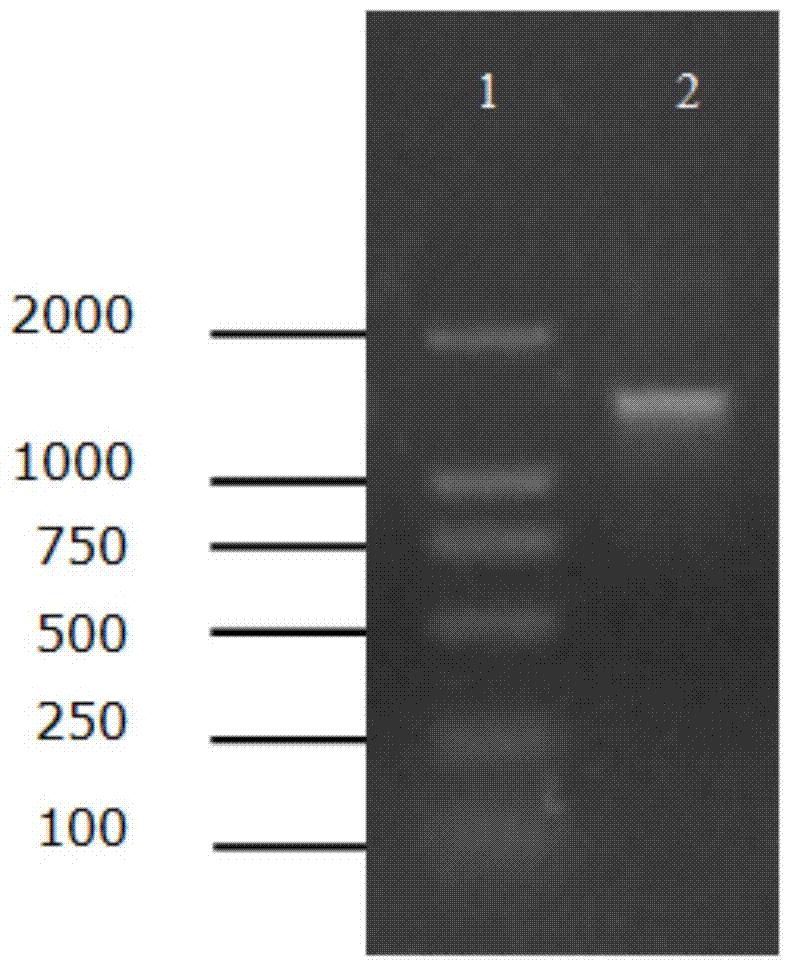
[0029] Example 1: Acquisition of D. tsuruhatensis ZJB-05174 amidase gene
[0030] The DNA extraction kit was used to extract the whole genome DNA of D. tsuruhatensis ZJB-05174 thalline, using the DNA as a template, primer 1 ( CATATG ACCCAAGCCCTCCCCCTGC), Primer 2 ( GAATTC TCAGCCCTGCGCCGAAGCC) was used as primer for PCR amplification reaction. The amount of each component in the PCR reaction system (total volume: 50 μL): 5 μL of 10×Pfu DNA Polymerase Buffer, 1 μL of 10 mM dNTP mixture (2.5 mM each of dATP, dCTP, dGTP, and dTTP), cloning primer 1 and primer 2 at a concentration of 50 μM 1 μL of each, 1 μL of genomic DNA, 1 μL of Pfu DNA Polymerase, and 40 μL of nucleic acid-free water. The PCR reaction conditions were as follows: pre-denaturation at 94°C for 5 min, followed by a temperature cycle of 94°C for 30 s, 56°C for 30 s, and 72°C for 1.5 min, a total of 30 cycles, and finally an extension at 72°C for 10 min, with a termination temperature of 4°C.
[0031] Take 50 μL ...
Embodiment 2
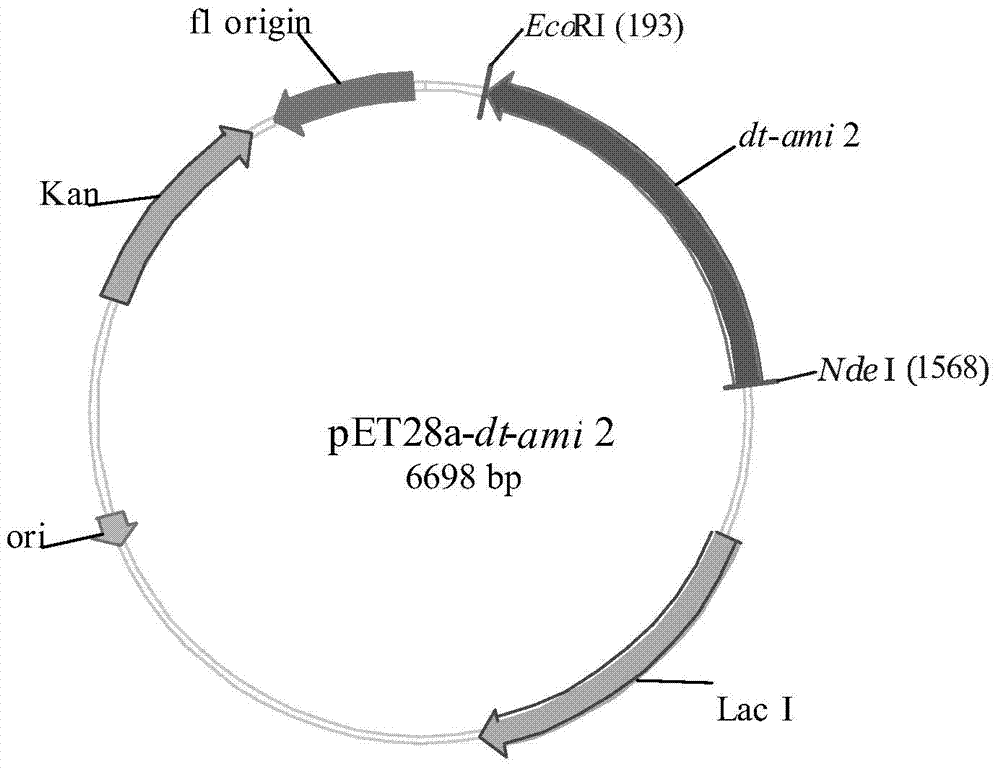
[0032] Example 2: Construction of recombinant expression vector pET28a-dt-ami 2
[0033] According to the analysis results of Example 1, the plasmid pMD18-T-dt-ami 2 was extracted using a plasmid extraction kit, and double-digested with restriction endonuclease NdeI / EcoRI (Fermentas), and recovered with T4 ligase (Promega ) Ligate the fragment with the commercialized vector pET-28a (Novagen) treated with the same restriction endonuclease overnight to construct the recombinant expression plasmid pET28a-dt-ami2.
Embodiment 3
[0034] Embodiment 3: Construction of engineering bacteria E.coli BL21(DE3) / pET28a-dt-ami 2
[0035] The recombinant expression vector pET28a-dt-ami 2 constructed in Example 2 was transformed into Escherichia coli BL21(DE3), spread on LB plates containing a final concentration of 50 μg / mL kanamycin, cultured overnight at 37°C, and randomly picked The clones were taken for colony PCR identification, and the positive clones were sequenced and verified. The results showed that the recombinant expression vector pET28a-dt-ami 2 was successfully transformed into the expression host E.coli BL21(DE3), and the amidase Dt-Ami 2 gene had been successfully cloned into pET NdeI and EcoRI sites at -28a.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com